Neurology is a baffling area. The diseases of the central nervous system and mental disorders are some of the most intriguing and complex diseases to resolve. Although the discipline of neurology was established 350 years ago, rapid progress has occurred in the last few decades as advances in technology provided the medium to further unravel the mysteries of the mind and the nervous system. But a lot remains unknown in this highly complex, interlinked, and broad field of neuroscience. The disorders are many and diverse, including prominent ones like Alzheimer's, schizophrenia, dementia, psychosis, epilepsy, insomnia, depression, Parkinson's, multiple sclerosis, and more. But the breakthroughs are hard to come by.
Rendering of Brain Neurons, Source: NIH
Beta-Amyloid Thesis
The beta-amyloid thesis that has been the underpinning of many major R&D efforts to cure Alzheimer's with billions spent, has been losing its appeal after a series of significant failures which have led pharmaceuticals companies to walk away. The hypothesis states that the buildup of the protein beta-amyloid in the brain is central to the onset and progression of Alzheimer's. Thus, it contends that you wash away the plaque and you reverse the Alzheimer's symptoms.
But the reality has not lived up to the promise thus far, with trials after trials falling short over decades. However, even though the beta-amyloid hypothesis teeters on the edge, it still clings on as some positive news rekindles interest and advocacy for the approach. The theory was given a lifeline in 2019 by Biogen's (BIIB) drug, Aducanumab, which achieved the rare distinction of both failing and then being claimed as a success in the space of six months. Aducanumab is being positioned by Biogen for mild improvement in cognitive functions for patients with Alzheimer's, and earlier this year the FDA deferred the PDUFA date from March to June 2021. The unprecedented set of dramatic events around this drug have not receded, with three members of the Advisory Committee, which had almost unanimously recommended a decline for Aducanumab last November, wrote in a JAMA op-ed earlier this month on why the drug should be rejected by the FDA. The Aducanumab decision is one of the most highly anticipated ones this year.
A couple of weeks ago, Eli Lilly announced that its drug Donanemab appears to have successfully slowed cognitive decline in patients with the early symptoms of Alzheimer’s and also reduced tau protein tangles in the brain, a predictive biomarker for the spread of Alzheimer's. The drug is based on clearing amyloid plaque, thus giving the hypothesis another lifeline and keeping the interest in it alive.
More Approaches To Be Considered
We're far from solving the scourge of Alzheimer's and dementia and other related mental disorders. With the amyloid thesis under question, there has been a clear yearning for new approaches. Robert Moir, a Harvard scientist, who offered quite different, and often characterized as radical, theories in Alzheimer's had postulated that the existence of beta-amyloid in the brain is for a reason, which is to defend it from infections. It's not the presence of the protein beta-amyloid that causes the disease, but likely the over-production. Thus, the research objective should be to manage its production. His work is no longer considered radical. Dr. Moir passed away in 2019.

Dr. Robert Moir
A team led by scientists at Scripps Research published the results of their recent study in the Proceedings of the National Academy of Sciences in late March 2021. It presented an insightful conclusion.
Scientists find evidence that antibody-based treatments in clinical trials for neurodegenerative diseases may trigger an inflammatory response in human brain immune cells, eroding their positive effects."
Thus, the antibody-based treatments designed to target the protein clusters found in Parkinson's disease and Alzheimer's disease were shown to worsen brain inflammation. For the first time, human brain cells were used in such a study instead of the mouse brain cells used in prior studies.
We see this inflammation in human microglia, but not in mouse microglia, and thus this massive inflammatory effect may have been overlooked in the past...Our findings provide a possible explanation for why antibody treatments have not yet succeeded against neurodegenerative diseases.” - Stuart Lipton, MD, Ph.D., co-senior author of the study and Founding Co-director of the Neurodegeneration New Medicines Center at Scripps Research.

Dr. Stuart Lipton
The aggregates or protein clusters are not only the large ones but also the much smaller clusters called oligomers that are now widely considered the most harmful to the brain, likely due to their inflammatory effect on healthy neuron cells.
Lipton noted the team has recently developed an experimental drug that may be able to counter this inflammation and thereby restore any benefit of antibody treatment in the human brain.
Numerous biotechs are pursuing innovative paths in CNS diseases. Some of these include Denali Therapeutics (DNLI), Ionis Pharmaceuticals (IONS), Neurocrine Biosciences (NBIX), Sage Therapeutics (SAGE), Arvinas (ARVN), Avadel Pharmaceuticals (AVDL), Supernus Pharmaceuticals (SUPN), Biohaven Pharmaceutical (BHVN), Intra-Cellular Therapies (ITCI), Karuna Therapeutics (KRTX), to name a few.
Below, we write on three small cap companies in the neurology space which investors can keep an eye on. They have lead compounds that could end up being a pipeline-in-a-product and have different approaches to pursuing therapeutics for Alzheimer's and Parkinson's diseases. Of course, there are many other neuro companies as well with innovative mechanisms of action.
Anavex Life Sciences
Anavex (AVXL) is developing its pipeline for a variety of CNS diseases using a precision approach by analyzing genomic data from clinical trials to identify biomarkers that can indicate the best patient fit for a therapeutic benefit.
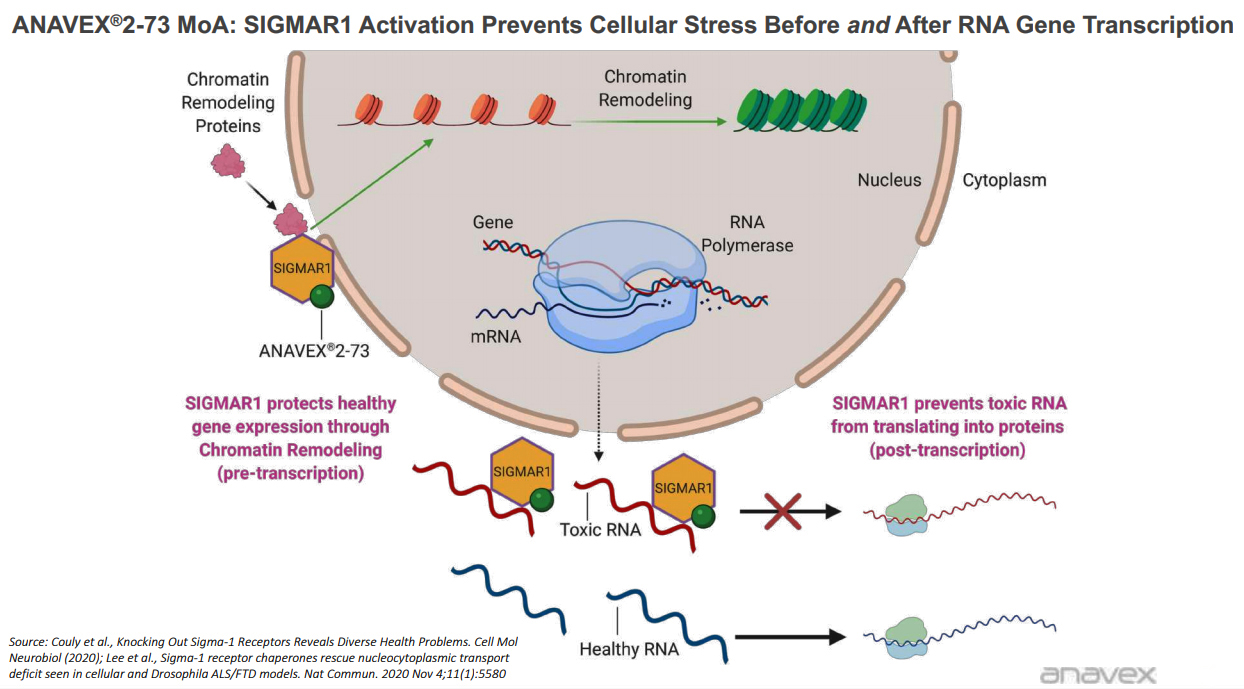
Anavex's approach is based on cellular homeostasis, which refers to a state of harmony or balance between various elements in and outside a cell. Maintaining a state of equilibrium keeps the cell healthy and allows for the normal functioning of the cells and the system they are part of. Chronic homeostasis imbalances or cellular stress is possibly caused by age-related buildups and may be responsible for many diseases. When defects arise in the homeostasis of proteins and RNA, it can lead to neuron dysfunctions. As the defective cells or their elements aggregate, they begin to spread and that is a characteristic finding in Alzheimer's and Parkinson's diseases where the nerve cells degenerate. The presence of amyloid plaques and neurofibrillary tangles are examples of such aggregates, which are typical of the pathology of Alzheimer's disease. With the SIGMAR1 activation through agonists, like its lead compound Anavex 2-73, the company believes that correcting the defects and restoring homeostasis in the brain region can potentially halt or delay neurodegeneration.
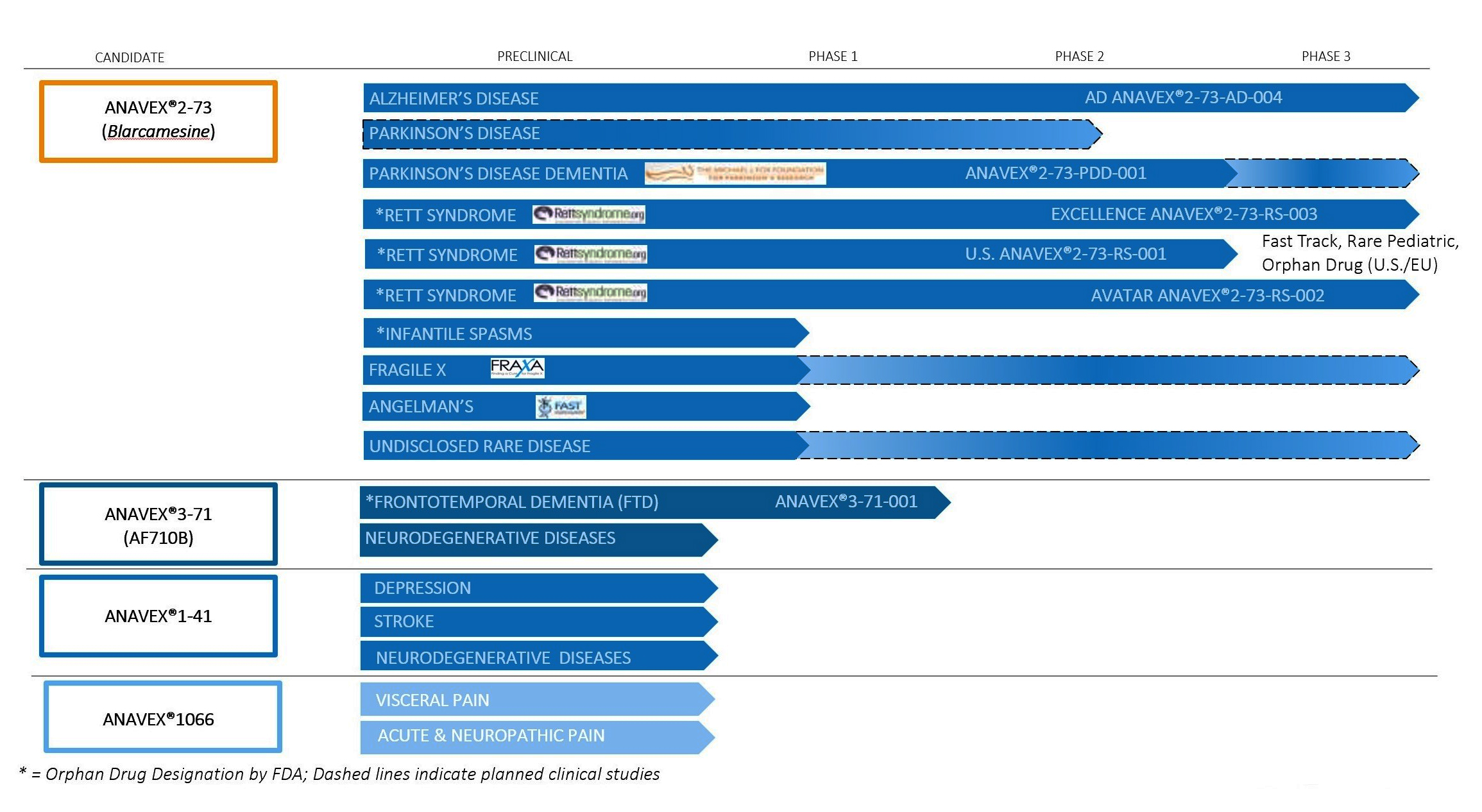
The Company's lead compound, Anavex 2-73 or Blarcamesine, is being developed for Alzheimer's and Parkinson's diseases, along with Rett Syndrome, a rare neurological disease.
The mechanism of action for the drug relies on targeting the sigma-1 and muscarinic receptors, which studies have shown to reduce stress levels within the brain, restore cellular homeostasis, and reverse the typical pathological biomarkers of Alzheimer's disease.
Anavex is matching the homeostasis theory with a precise approach to identifying patient groups that can best benefit. A genomic analysis of Alzheimer's disease patients treated with Anavex 2-73 allowed the company to identify key genetic variants, and study the impact of the drug on a memory function gene called COMT. The company believes that using genetic biomarkers, identified from within the study population, to target patient groups is likely to generate a more robust response in patients with Alzheimer's disease.
Anavex owns the global rights to the entire pipeline, and has multiple ongoing trials, with a few of them in Phase 2 and 3. The lead compound resembles a pipeline-in-a-product and is being tested for Alzheimer's, Parkinson's, and Rett Syndrome. This year there are multiple readouts from Phase 2 and Phase 2/3 trials of both topline and complete clinical data.
The series of data to be released in the first half should clarify the potential of the SIGMAR 1 activation pathway and confirm if the positive data from Anavex so far can lead to something more promising.
Cassava Sciences
Cassava Sciences (SAVA) is focused on a key therapeutic candidate targeting neurodegenerative diseases. The compound, Simufilam, is an oral, small molecule leading candidate that binds with high affinity to altered filamin A (FLNA), and contributes to restoring its normal shape. FLNA is a scaffolding protein, one that binds two or more proteins to create a functional unit, and is widely found throughout the body. Misfolded or altered FLNA in the brain has been shown in published studies to cause neurological dysfunction, degeneration, and inflammation.
Such an altered form of FLNA protein is found in a brain affected by Alzheimer’s disease. In Alzheimer’s, proteins can lose their healthy shape and functionality, and break down. Once they break down, these proteins coalesce in clumps or clusters to form plaque. Amyloid plaque is formed when amyloid proteins break down. Clearing such protein clusters, also called aggregates, has been the thrust of most key therapeutic approaches over the past decades, including the beta-amyloid thesis.
By returning the protein to its normal conformation and stabilizing it, Simufilam is being developed to restore the normal functioning of key brain receptors that play pivotal roles in brain cell survival, cognition, and memory, and arrest the toxic effects of the misfolded FLNA. It is important to note that the mechanism of action relies on stabilizing the protein and not removing amyloid from the brain, something that Dr. Moir's research, mentioned above, had pointed to.
Simufilam also has a strong anti-inflammatory effect by reducing cytokines in the brain. This is critically important. The combination of restoring key brain receptors along with a powerful anti-inflammatory benefit can be an important differentiator in delivering sustainable positive outcomes. The anti-inflammatory characteristic in brain-related neurological treatment was the direction that the recent study led by Scripps Research scientists, mentioned earlier, pointed to as being a potentially positive pathway for a therapeutic.
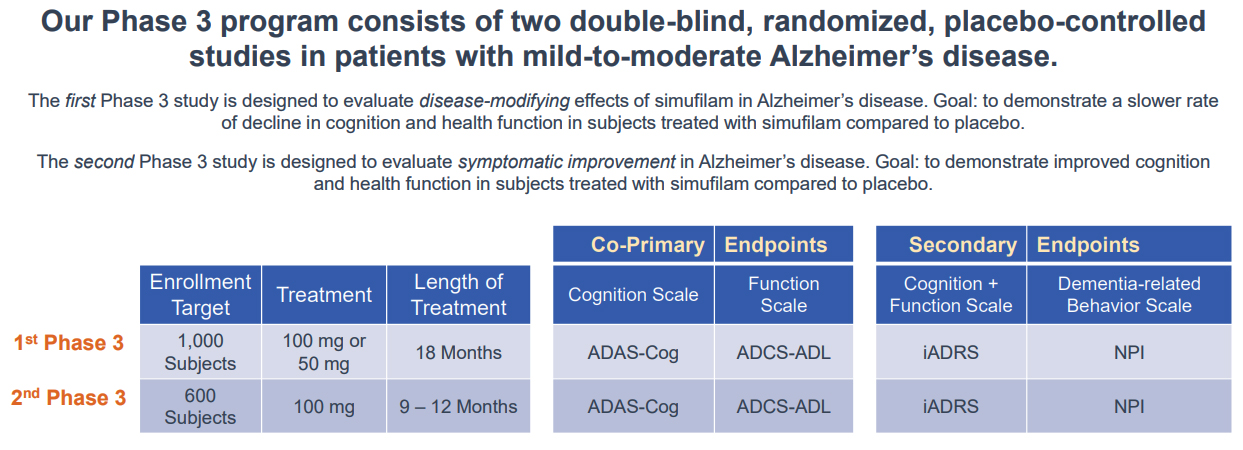
In early February, the Company announced the interim analysis of the open-label study of Simufilam for patients that have been treated for at least 6-months. The treatment improved cognition scores by 1.6 points, a 10% mean improvement from baseline to month 6. The group also showed an improvement in dementia-related behavior, by 1.3 points, a 29% mean improvement from baseline to month 6. Notably, the compound was able to not only arrest cognitive decline but even improve it. The next planned interim analysis will be reported around mid-2021 and will detail clinical data on the first 50 patients who reach the 12-month mark. In late February, the Company announced the completion of the End-of-Phase 2 meeting and defined the key elements for a pivotal Phase 3 program to support a New Drug Application (NDA). The trial is expected to begin in the second half of 2021.
Cassava's pipeline is narrow. But the strong multiple effects of the Simufilam compound and its huge pipeline-in-a-product potential can offset such concerns if the investigational drug is successful in Phase 3. At this point, it is the outcome of Simufilam that will determine the future of Cassava Sciences.
Prothena
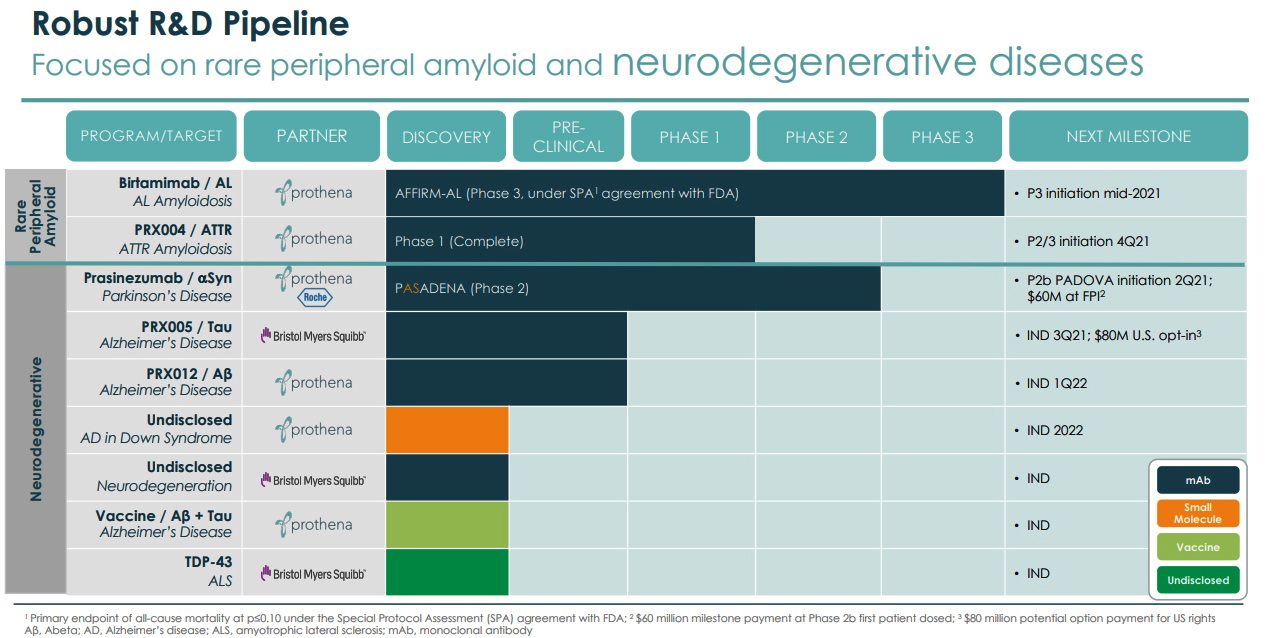
Prothena (PRTA) has a pipeline of fully-owned and partnered programs that are focused on neurodegenerative diseases, like Alzheimer's and Parkinson's, leveraging its expertise in protein dysregulation and understanding the biology of misfolded proteins and neurological dysfunction.
Its lead program is the wholly-owned Birtamimab for the potential treatment of AL Amyloidosis, a rare disease. Birtamimab is an antibody designed to neutralize toxic aggregates and promote clearance of amyloid in patients. The mechanism of action targets misfolded kappa and lambda light chain to clear deposited amyloid that causes organ dysfunction and failure. In Feb 2021, the company had announced that after FDA meetings following Phase 2, it is expected to begin Phase 3 trial in the second half of this year. The study, AFFIRM-AL, will be a double-blind, placebo-controlled, time-to-event trial that is expected to enroll about 150 newly diagnosed, treatment naïve patients. Birtamimab has been granted Fast Track and Orphan Drug designations.
Prothena's next leading program, Prasinezumab, is partnered with Roche for early Parkinson's disease. Prasinezumab is the first potentially disease-modifying antibody to demonstrate signals of efficacy on multiple pre-specified clinical endpoints in patients with early Parkinson’s disease, and the first anti-alpha-synuclein antibody to advance into late-stage development.
Alpha-synuclein is a protein found in neural tissue that can aggregate and spread from cell-to-cell resulting in dysfunction and loss of neuronal activity. There has been a growing body of evidence that alpha-synuclein can be propagated and transmitted from neuron-to-neuron, resulting in the spreading of neuronal death, and such neurodegeneration can be diminished by targeting the errant forms of the protein. This is the focus of Prothena's antibody Prasinezumab.
In the Phase 2 study, Prasinezumab significantly reduced the decline in motor function by 35% versus the placebo on the Parkinson’s Disease Rating Scale after a year of treatment. The two companies will be advancing the investigative treatment into Phase 2b PADOVA study in the second quarter of 2021. Prothena will earn a $60 million clinical milestone payment upon the dosing of the first patient. The licensing agreement can allow Prothena to receive up to an aggregate of $600 million in upfront and milestone payments from Roche, of which $75 million has already been received, and a 70/30 split between Roche and Prothena for all costs and profits.
The company has a few other early-stage programs in neurology, including Alzheimer's disease. Prothena was formed in Ireland and was spun-off from Elan Corporation in 2012.
Biotechs Under Pressure
Biotechs have been under pressure since early February as interest rate concerns have overwhelmed the industry group and caused a sharp retreat. The biotech indexes have declined sharply to their long-term trendline and have negative returns for the year. As of April 1, the Nasdaq Biotech Index (IBB) was down -2%, and the S&P Biotech Index (XBI) over -5%.
Rising expectations for inflation can lead to persistently rising interest rates, which hurt growth companies, particularly unprofitable ones like biotechs, as their future earnings are eroded faster by higher rates. That concern has been the proverbial wet blanket over the biotech industry group and holding back the healthcare sector which is now looking poised to break out after a 3-month consolidation. Interest rates play a vital part in biotech valuations as highlighted in the 2021 Biotech Outlook article. Even though there is a macro-economic issue weighing down valuations, we believe biotechs are well-positioned. It is hard to anticipate the timing of a turnaround, but it will be just as sharp when it occurs. In the Prudent Healthcare model portfolio, which was up +25% compared to the S&P Healthcare index gain of +3% as of April 1, we are fully invested with a 40% biotech exposure.
A few promising biotech companies, some of which may be now or in the past part of Prudent Healthcare or Prudent Biotech model portfolios, include Moderna (MRNA), Agios Pharmaceuticals (AGIO), Vericel (VCEL), MacroGenics (MGNX), Anavex Life Sciences, Cassava Sciences, Prothena, Aclaris Therapeutics (ACRS), Morphic (MORF), TG Therapeutics (TGTX), Horizon Therapeutics (HZNP), Curis (CRIS), Essa Pharma (EPIX), Rubius Therapeutics (RUBY), Intellia Therapeutics (NTLA), and Celldex (CLDX).
The article was first published on Seeking Alpha.