Biotech Pulse
As 2020 has slipped into the rear-view mirror, it will not be a misnomer to refer to it as the year of the Biotechs.
The biotech industry was on a mission to return the pandemic ravaged world to normalcy by delivering a cure. The industry has succeeded by already delivering two vaccines for COVID-19 and treatments, with more to come as the new year begins, putting the world on a path to recovery. The monumental impact of the biopharma effort cannot be overstated. Lives will be saved, livelihoods restored, and economies rebuilt.
The Nasdaq Biotechnology Index (IBB) also achieved a major milestone during the year as it finally made a new high - five years after the one recorded in July 2015. It was a pivotal moment whose breakout gains will be achieved in 2021 and beyond.
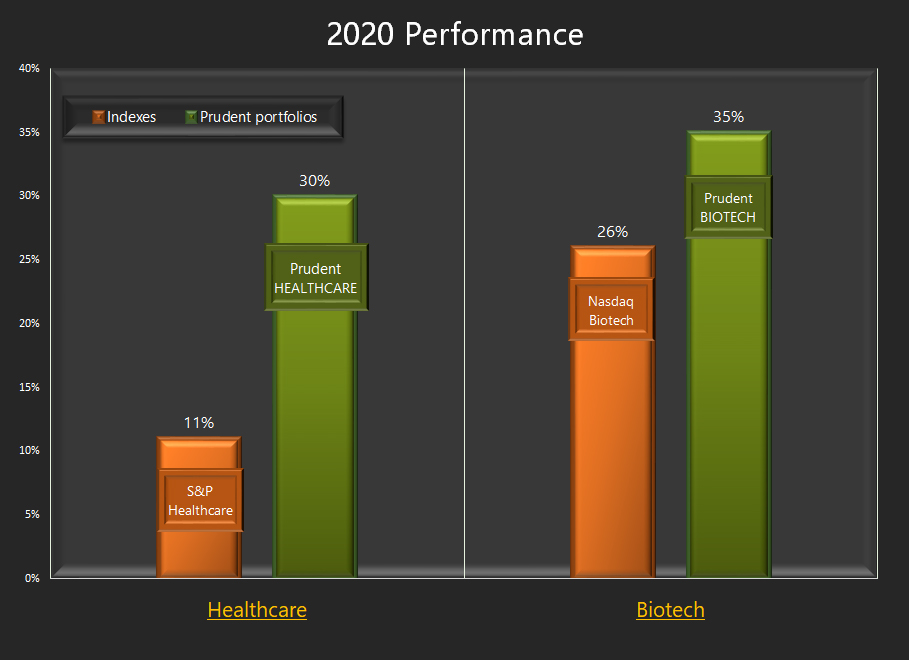
In the 2020 outlook, we had expected the Nasdaq Biotechnology Index to be up +10% and raised it to +20% in the mid-year update. The premier Biotechnology Index rose +26% in 2020.
The year presented challenges that were unprecedented in modern history. So predicting the market was a game of luck! You could be right one month and be completely wrong the next.
In determining total returns, equally important as security selection are the times when one is invested and the investing behavior based on the investment approach. The approach served us well as the various Prudent model portfolios outperformed their benchmarks.
2020 Biotech, Healthcare and Smallcap Performanc
Vaccines - It Doesn't Get Bigger For Biotechs...
A compelling product in a captive market worth billions of dollars is an incredible opportunity for any company. The world has to be vaccinated fast to emerge from this slow-motion tragedy. A tragedy that hasn't seen its worst moments yet, as the fatality rate will likely peak in February if the pandemic follows the trajectory of the flu season.
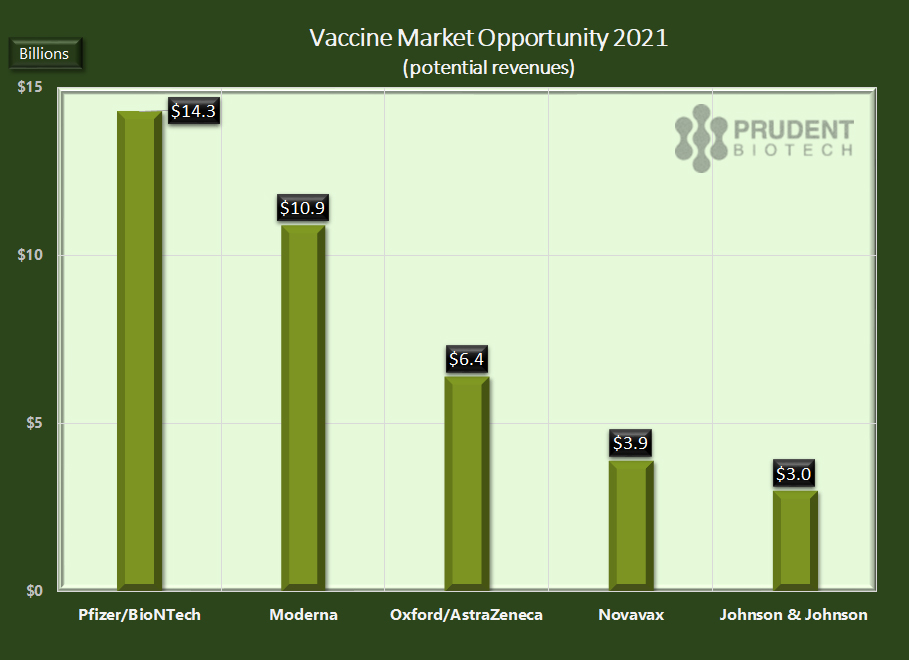
AllianceBernstein (AB), a financial services firm, has estimated the market to be nearly $40 billion in 2021. And the first-mover advantage will obviously be tremendous in the first-year. Premium pricing will exist in the supply agreements during the first half due to scarcity and then trend down rapidly in the second half as more lower-cost vaccines ramp up production. As demand ebbs in developed countries once first-year supplies are secured, lesser-developed countries will be served at lower pricing. The following year 2022 would likely need a new vaccine, just like the flu one, thus creating a smaller but recurring market opportunity with a lot of innovation potential. Note that there is no data yet on the longevity period for the vaccine and thus how frequently it would be needed - every year, every 2 years, etc
In 9-months the biotech industry delivered 2 vaccines approved under an Emergency Use Authorization (EUA). The first 2 vaccines, BioNTech (BNTX) with partner Pfizer (PFE) and Moderna (MRNA), raced to the finish line using a novel messenger RNA (mRNA) approach. Untested and unproven clinically against any disease thus far, this highly-suitable vaccine development approach has delivered for the world
Is the high efficacy rate the result of the mRNA approach or the ease of targeting the spike protein?
It's a highly interesting question whose preliminary answers will be available this month in January.
It has been truly amazing to record a 94% to 95% efficacy or effectiveness rate in early trial results for mRNA vaccines. This sets a high bar for other vaccines using more traditional approaches. Nearly all of them target the spike protein on the COVID-19 virus, just like the mRNA vaccines, and thus should have good effectiveness, well north of 50%. FDA has set a 50% efficacy benchmark for EUA approval.
RNA vaccines have already demonstrated a premium position based on up to 95% efficacy in early Phase 3 trial results. This would suggest that similar efforts using the mRNA approach, particularly from companies like CureVac (CVAC), can likely lead to similar results. Although, it must be observed that simply working on an RNA vaccine doesn't mean that the same 90+% efficacy level will be achieved.
Oxford University and AstraZeneca's (AZN) product can quickly become a global vaccine if results are around the 70% level. The world's largest vaccine manufacturer, The Serum Institute of India, will be one of the biggest producers of the Oxford/AstraZeneca vaccine. The AstraZeneca vaccine trials, which have been plagued by befuddling issues and amateurish data handling, had an average efficacy rate of around 70%, but unfortunately, the results are not considered reliable and robust. The genetically engineered viral vector in the vaccine, developed by Oxford University, is deemed to be the most promising for the world due to its low-cost and the effectiveness of a similar approach against Ebola. The Oxford/AstraZeneca trial continues and more reliable results are expected this month in January. Novavax (NVAX) is expected to report trial results in late January, and one of its manufacturing partners is The Serum Institute. Finally, Johnson & Johnson (JNJ) is expected to roll out its vaccine results in late January.
These three trial results in January will provide efficacy data that will answer the question if mRNA vaccines can also have higher efficacy as a major advantage besides rapid development.
Internationally, Russia has a traditional viral-vector vaccine, Sputnik V, which it claims has 92% efficacy, but it doesn't yet have reliable public data. Sinovac, a Chinese firm, is also using the tested traditional approach of an inactive viral particle in the vaccine and released its early data from the Brazilian trials showed a 78% efficacy rate, though details remain missing. Its peer Sinopharm also has vaccines with the same traditional approach in late-stage trials, with a 79% efficacy rate, but again details are sketchy and conflicting. Both companies had vaccinated over a million people in China under emergency use, prior to Phase 3 results.
...But The Market Opportunity May Not Exist For All Players in 2021
Pfizer/BioNTech and Moderna are anticipated to be the largest beneficiaries of vaccine sales in 2021 due to their first-mover status with compelling and most likely best-in-class products. They will be followed by AstraZeneca, Novavax, and Johnson & Johnson, as per the AB research report. There will be much smaller pickings if at all for others, at least in the US.
A number of companies can receive FDA approval if the product achieves higher than 50% efficacy, but run the risk of not being able to scale up supplies in 2020. Most of the prominent beneficiaries of the market opportunity have received significant US government funding or have the financial wherewithal to secure raw materials, scale-up manufacturing, or enter into manufacturing alliances to ramp-up production. That is not true for all the vaccine developers, and most of them may get sidelined, even with approved products, from participating in the 2021 opportunity.
One would also believe that as more data becomes available, FDA can reconsider guidelines set during an emergency authorization, and set a higher vaccine efficacy rate for the future, thus potentially diminishing the market opportunity for relatively lower efficacy products.
Biotechs Are Well-Positioned For A Strong 2021
The year 2021 begins with biotech fundamentals well-positioned for significant gains. The macro-environment, scientific gains, and M&A activity continue to remain key drivers of biotech valuations.
Macro Environment Supportive Of Risk-Taking
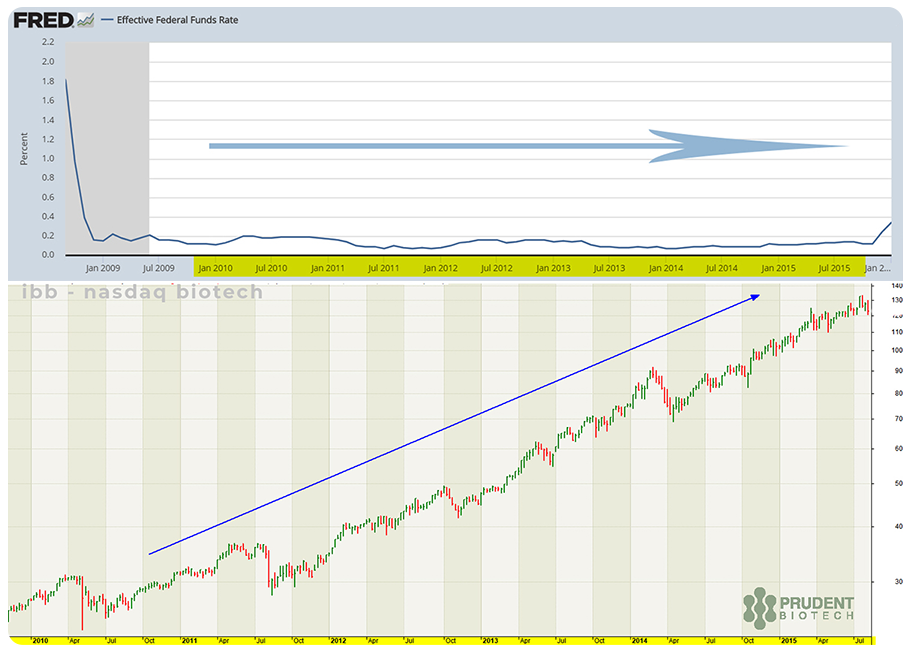
Biotechs are considered a speculative group, and such groups do particularly well in a prolonged low-interest-rate environment when risk-taking appetite is strong due to (a) low borrowing costs, and (b) low returns on interest-rate instruments. Smallcaps are also beneficiaries of such a market climate.
Biotech funding continues to soar at the venture and public level, and over one-third of the IPOs on the US markets in 2020 were of biotech companies.
We had presented the charts below in early 2020, illustrating the potential correlation between sustained low-interest-rates or low-cost-money and biotech valuations. The best period for biotechs during the past decade (2010-2019) was the ~6-year period from 2010 to 2015 when the Nasdaq Biotech Index soared nearly 400%. This was a period of a very low and stable interest rate policy.
The biotech indexes have broken out again after a prolonged consolidation. Even though there may not be a perfect causal relationship between interest rates and biotech performance, at the very least, the correlation underscores the existence of a favorable macro environment for biotechs that should continue in the years ahead, and sustain a longer-term, multi-year uptrend.
Scientific Advances Keep Coming
The rapid development of RNA vaccines has underscored the advancing frontier of science where technology and discovery are advancing at a relentless pace, generating new treatments in a shorter time.
RNAi is now a highly prized and promising approach for drug and vaccine development, after decades of false-and-slow-starts. The space has strong momentum as it has captivated the world with Moderna and BioNTech's rapid COVID-19 vaccine development, and illustrates the rapidly advancing frontiers of drug development. RNAi medicines work upstream, almost at the pre-genetic level, then most therapeutics, and thus the gene silencing approach possesses transformational potential.
mRNA is aptly suited for vaccines. It is not hard to understand why, if one imagines working upstream at a genetic level to build defenses against antigens. Moderna and BioNTech were already focused on vaccines for various diseases including cancer vaccines, as opposed to therapeutics or drug treatments. As the speed of development and efficacy rate of COVID-19 mRNA vaccines has shown, this is a breakthrough platform and no longer a concept.
Alnylam Pharmaceuticals (ALNY) has led the charge in RNAi therapeutics when its drug Onpattro was approved in 2019, the first RNAi drug to receive the FDA blessing. It now has a growing stable of approved RNAi drugs, including the cholesterol drug Inclisiran (renamed Leqvio) which is now owned by Novartis. Arrowhead Pharmaceuticals (ARWR) has a number of meaningful programs with data readouts this year. A few others in this growing field include Dicerna (DRNA), Translate Bio (TBIO), and Arcturus Therapeutics (ARCT).
Gene editing continues to transform medicine not only for genetics-based diseases but also in search of new drug discoveries for old maladies. Clinical trials of genome editing agents including CRISPR editors and zinc finger nucleases continue to grow. The list of players is ever-expanding from Crispr Therapeutics (CRSP), which has a partnership with Vertex Pharmaceuticals (VRTX), Editas Medicine (EDIT), and Intellia Therapeutics (NTLA), being a few. A number of smaller public and private companies are using variants of CRISPR gene-editing to make the approach more robust, precise, and less risky. Last month, Eli Lilly acquired Prevail Therapeutics (PRVL), a gene therapy player in the neurology area.
CAR-T cell therapies in oncology are advancing rapidly and also broadening out to include more clinical trials of allogeneic products, which are off-the-shelf cells as opposed to autologous products that are unique to the patient, that reduce manufacturing challenges and significantly reduce costs. There are more than 600 clinical trials ongoing in CAR-T cell therapies. Management consulting firm McKinsey forecasts CAR T-cell therapies to go from less than $1 billion in global revenues a year ago to over $10 billion in 2024.
Oncology Remains The Leading Investing Area and Neurology The Most Baffling
The fight for a cure for cancer continues with Oncology receiving the most funding in private and public financings, and the most M&A related deal activity, by far. Neurological diseases and rare diseases are also high on the list of attracting investing dollars.
Advances on major neurological diseases, like Alzheimer's and Parkinson's, continue to be difficult and rigorous, and it's an area where progress appears to be defined more by learning what doesn't work. The beta-amyloid hypothesis is a perfect illustration. The theory postulates that the protein creates plaques in the brain, causing Alzheimer's, and thus its reduction can prevent or rollback the disease. After decades and billions of dollars spent in research, each outcome has just proven this hypothesis to be wrong, and some large players have exited from this approach. Biogen (BIIB) has continued to go down this path and may receive key approval in January for its drug Aducanumab to treat mild-Alzheimer's. If granted, it will be the first approved treatment for Alzheimer's disease and a material valuation enhancing event for biotechs.
Strengthening M&A Activity
Biotechs are amongst the most active M&A industry groups. That's the nature of the industry - positive outcomes in mid-and-late-stage trials, particularly in oncology, can elicit strong interest from pharmaceuticals.
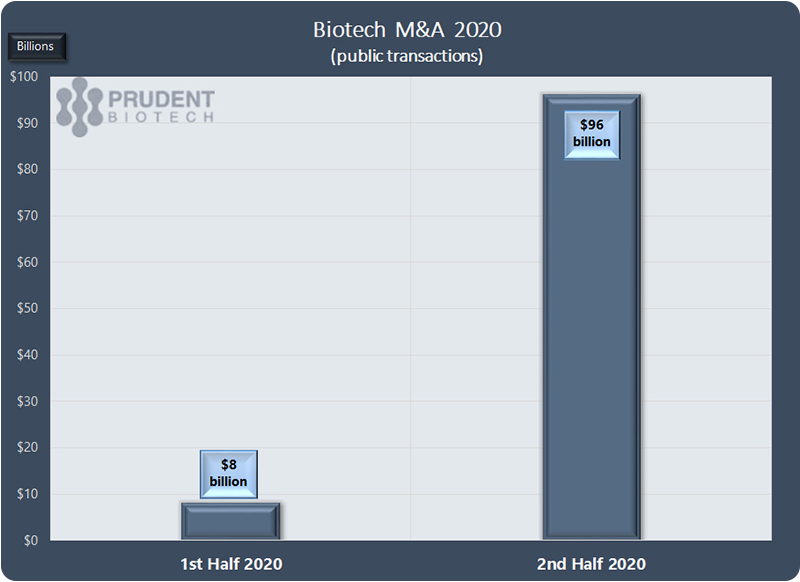
As the pandemic struck, the M&A activity ebbed rapidly and in the first half of 2020 less than $10 billion of transactions were done. This changed in the second half as nearly $100 billion of deals were recorded.
A significantly more normalized environment in 2021, as the pandemic's grip weakens, low-cost money, and declining returns on internal R&D for big pharmaceuticals, are going to be highly supportive of M&A activity.
Regulatory Environment
The drug pricing issue, dormant for now, will re-emerge later in 2021 as the pandemic subsides. It may come to the forefront briefly this month as the companies put through the annual drug price increases in January. So far this month, prices for nearly 600 drugs have been raised by an average of 4.2%. This compares to January price increases for 639 drugs by an average of 6% in 2020.
There have been efforts in the waning days of the Trump Presidency to introduce new Executive Orders (EO) to reset prices. In late November, the administration had announced a policy to base the Medicare prices for 50 drugs on the lowest price paid by a group of developed nations. Another policy was aimed at eliminating drug rebates paid out to middlemen by drug companies. These policies are vulnerable to legal challenges from the industry, which is against any type of price control and indexing. It is unclear how the incoming Biden administration will deal with the EOs.
It can't be denied that healthcare costs are the number one public interest issue. However, the issue enjoys bipartisan support only in principle, and the paths to address healthcare costs being proposed by the two parties are poles apart. With Democrats now holding a thin majority in Congress, sweeping changes may not be possible. One would believe there will be meaningful moves, but incremental ones. Thus, the needle may not move meaningfully at least during 2021, and the impact on the healthcare industry will be restrained.
However, the threat of greater regulation is an overarching risk with the potential to upend the industry dynamics eventually in the years ahead.
Outlook
A young bull market operating in a market environment of benign monetary policy and trillions of fiscal stimulus will continue to attract money to the higher-risk and more volatile segments of the market like biotechs.
Fundamentally, biotechs continue to be well-positioned based on promising scientific advances. Technically, a breakout after a 5-year, long-term consolidation augurs well for biotechs and promises sustained gains over 2021. We anticipate the Nasdaq Biotech Index to record another 20+% return in 2021. The return expectation is simply a general framework and will need to be adjusted if market conditions deviate significantly from current expectations.

There will be meaningful opportunities to materially outperform the indexes. But there will be times when reducing the exposure may be best, based on the investment approach. Biotechs being a high risk-reward industry, the risk management part is quite important to build returns over time. Investors need to pursue a concrete investment strategy in biotechs, preferring a portfolio approach by investing in a basket of promising biotech companies that can assist in managing risk and overcoming mistakes that do happen.
A couple of key events to watch for in January would be the J.P. Morgan Healthcare Conference from January 11 to 14, which helps healthcare valuations, and an FDA decision on Aducanumab.
A few promising biotech companies, some of which may be now or in the past part of Prudent Healthcare or Prudent Biotech model portfolios, include Moderna, Allakos (ALLK), Denali Therapeutics (DNLI), Arrowhead Pharmaceuticals (ARWR), Fate Therapeutics (FATE), Crispr Therapeutics, Rocket Pharmaceuticals (RCKT), TG Therapeutics (TGTX), Arvinas (ARVN), Kodiak Sciences (KOD), BridgeBio Pharma (BBIO), Five Prime Therapeutics (FPRX), Beam Therapeutics (BEAM), Intellia Therapeutics (NTLA), Precigen (PGEN), Scholar Rock (SRRK), Trillium Therapeutics (TRIL), and Editas (EDIT).
Industry exposure can also be acquired through ETFs like IBB, which tracks the Nasdaq Biotech Index, and XBI which tracks the S&P Biotechnology Select Index. Biotech investing is volatile and high-risk. Investors should pursue a concrete investment strategy preferring a portfolio approach by investing in a basket of promising companies that can assist in managing risk and overcoming mistakes.
The article was first published on Seeking Alpha.