Healthcare Pulse
Nuvalent, Inc. (NASDAQ:NUVL) is a clinical-stage biopharmaceutical company focused on developing precisely targeted therapies for cancer patients. Leveraging expertise in chemistry and structure-based drug design, Nuvalent is creating small-molecule drugs to overcome the limitations of existing treatments for clinically proven kinase targets. The company has two clinical targets in Phase 2 and another in Pre-Phase 1.
Kinase Inhibitors - A Brief Primer
Kinases are enzymes that add phosphates to other molecules in a cell, such as sugars or proteins, activating or deactivating them. Human cells have various kinases that help control cellular signaling, metabolism, division, and survival. Some cancer treatments target certain kinases that are linked to cancer.
Kinase inhibitors are substances that block kinase enzymes. Certain kinases are more active in some types of cancer cells. Blocking these kinases through inhibitors can prevent the cancer cells from growing, and inhibit the growth of new blood vessels in tumors.
Despite their potential in precision medicine, kinase inhibitors face challenges as their clinical utility is often hindered by resistance, off-target effects, and limited brain penetrance of current therapies in cases of brain metastases.
Kinase Resistance: Cancer cells frequently mutate, leading to resistance against current kinase inhibitors. Also, inhibitors can trigger new mutations in such cells, making them resistant.
Kinase Selectivity: The drug binding sites of different kinases are structurally very similar, which makes it challenging to develop molecules that inhibit only a single kinase target without affecting others and in turn causing major adverse events and reduced efficacy.
Nuvalent Drug Pipeline
Nuvalent designs small molecule drugs with structures that target both original and mutated kinases in drug-resistant cancer, aiming to treat tumors and emergent mutations while reducing kinase resistance. The company's focus on target selectivity helps overcome the challenges of kinase resistance, kinase selectivity, and brain penetration.
NVL-520 (Zidesamtinib) for ROS1 positive NSCLC
ROS1 fusions are an oncogenic aberration found in up to 3% of patients with non-small cell lung cancer (NSCLC). A ROS1 fusion or ROS1 rearrangement occurs when the ROS1 gene fuses with part of another gene, thus activating the ROS1 gene in a manner that causes cancer. Existing therapies for ROS1-driven NSCLC are limited by resistance mutations and central nervous system or CNS disease.
Nuvalent's NVL-520 (Zidesamtinib) is being developed for patients with ROS1-positive NSCLC. It is a novel ROS1-selective inhibitor and is designed to address the challenges of:
Treatment resistance to current ROS1 inhibitors and avoiding
inhibition of the structurally-related tropomyosin receptor kinase (TRK) family.
CNS-related off-target adverse events.
Limited brain penetrance in brain metastases, that limit the efficacy of current ROS1 tyrosine kinase inhibitors (TKIs).
Clinical Insights NVL-520
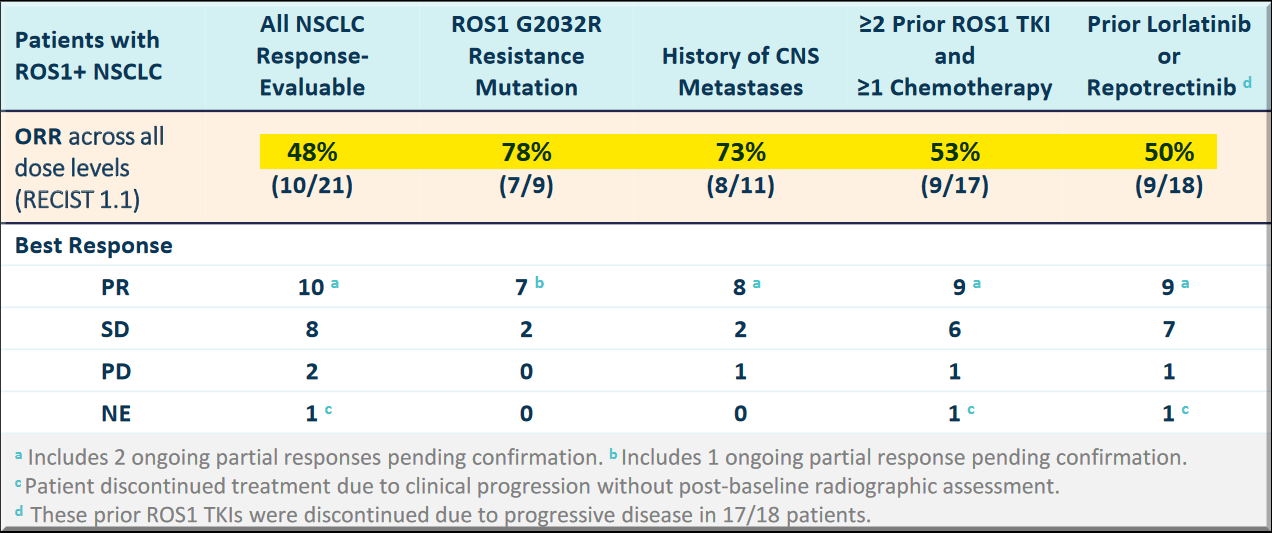
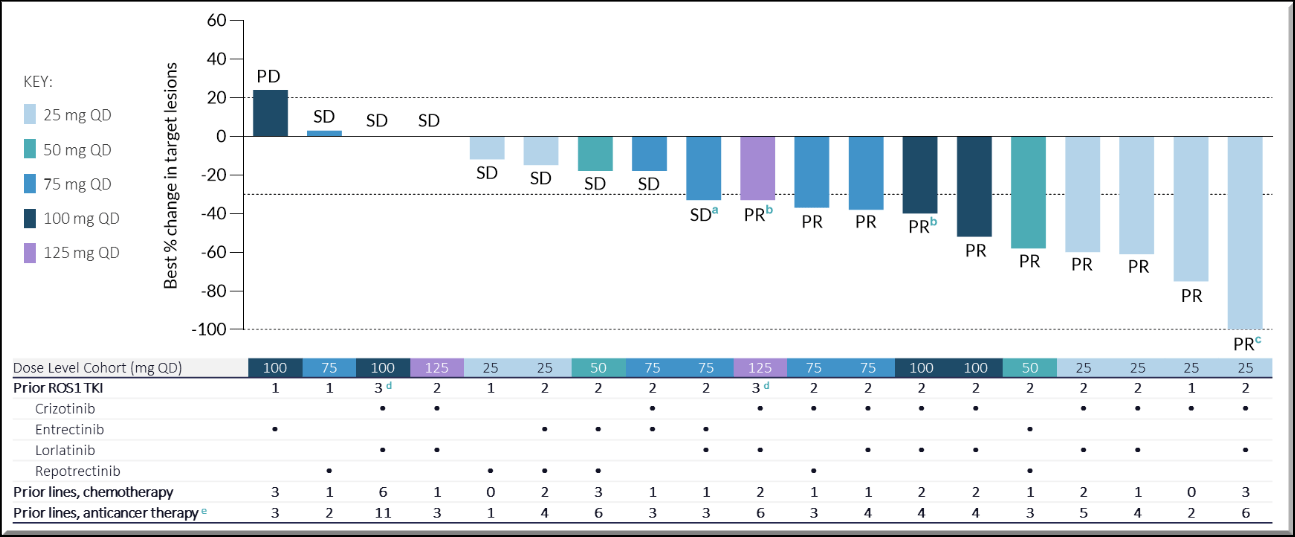
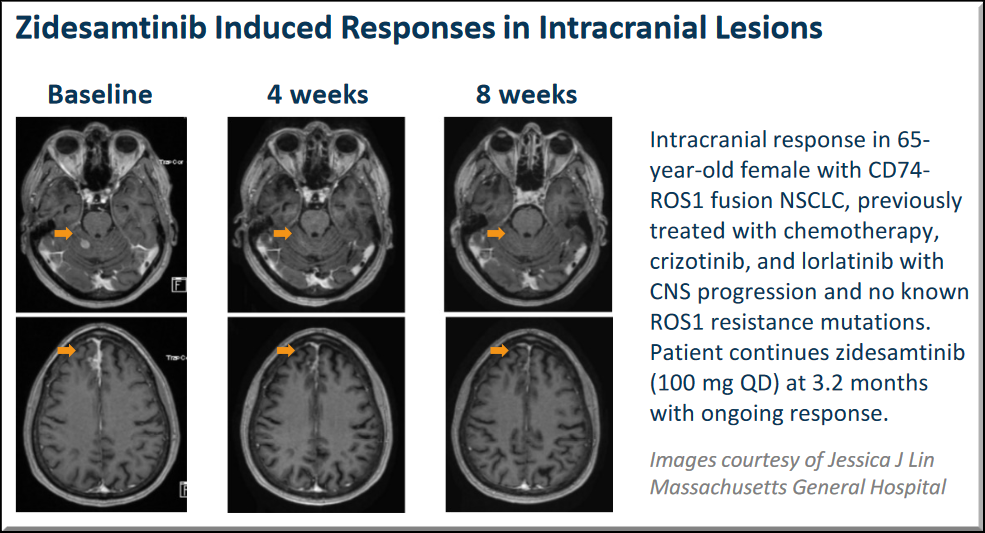
This is Nuvalent's first lead candidate and has reported promising results in the Phase 1 trial, where it induced a tumor response in a heavily pre-treated patient group. Safety and pharmacokinetic profiles were favorable and there were no treatment-related significant adverse events (TSAEs), and no adverse events (AEs) leading to dose reduction or discontinuation.
Tumor regression was observed across all dose levels of Zidesamtinib.
No CNS progression was observed at the time of data collection in any of the 35 patients. Intracranial partial response in lesions was observed in all the 3 patients with measurable CNS metastasis.
The Phase 2 portion of the ARROS-1 clinical trial began enrolling patients in September 2023, aiming to evaluate NVL-520's overall activity at the recommended dose of 100 mg QD and support potential registration for ROS1-positive NSCLC patients. The primary endpoint is objective response rate (ORR) by blinded, independent central review, and the secondary endpoints include efficacy measures, intracranial activity, and overall safety and tolerability.
During the second half, the company is expected to share updated data from the ARROS-1 Phase 1/2 trial.
NVL-655 for ALK-Positive NSCLC
ALK fusions occur in up to 5% of NSCLC patients. The anaplastic lymphoma kinase (ALK) gene contributes to the development of the gut and nervous system, while the baby is in the womb and then gets turned off in the womb itself. In some people, the gene gets turned back on and fuses with another gene. This alteration is called an ALK fusion and can result in an oncogene causing ALK-positive (ALK+) cancer. Current therapies for ALK-driven NSCLC are hindered by resistance mutations and CNS disease.
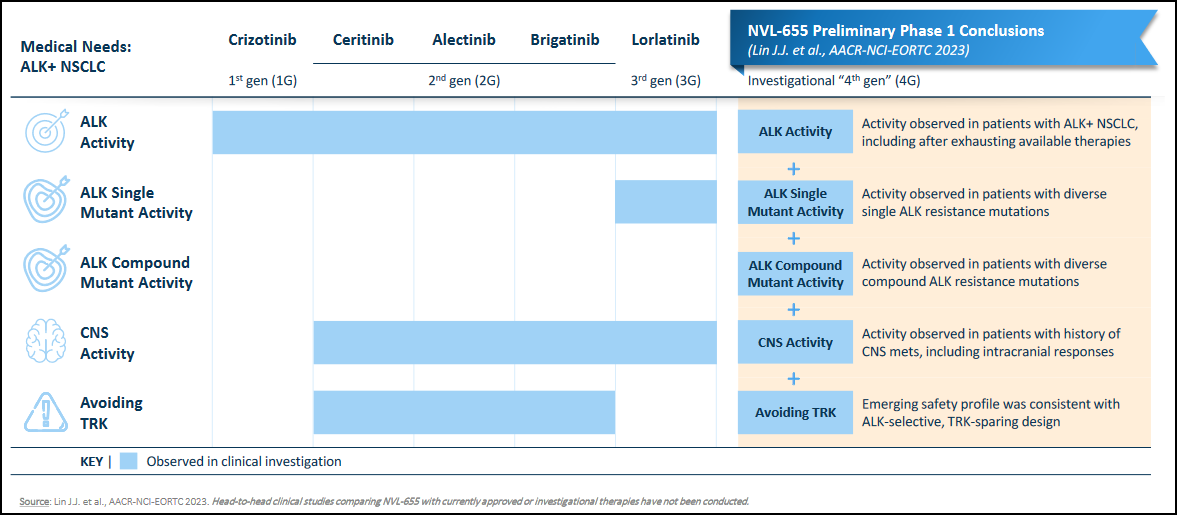
Nuvalent's second lead candidate, NVL-655, is a brain-penetrant, ALK-selective inhibitor designed to overcome treatment resistance, CNS-related adverse events, and brain metastases, which limit the existing first (crizotinib), second (ceritinib, alectinib, or brigatinib), and third (lorlatinib) generations of ALK inhibitors. It selectively inhibits, thus avoiding the inhibition of the related tropomyosin receptor kinase family and, consequently, minimizing CNS adverse events. NVL-655 has received FDA Breakthrough orphan drug designation for ALK-positive NSCLC.
NVL-655 has received the FDA's designations of Breakthrough Therapy and orphan drug designations for ALK-positive NSCLC.
Clinical Insights NVL-655
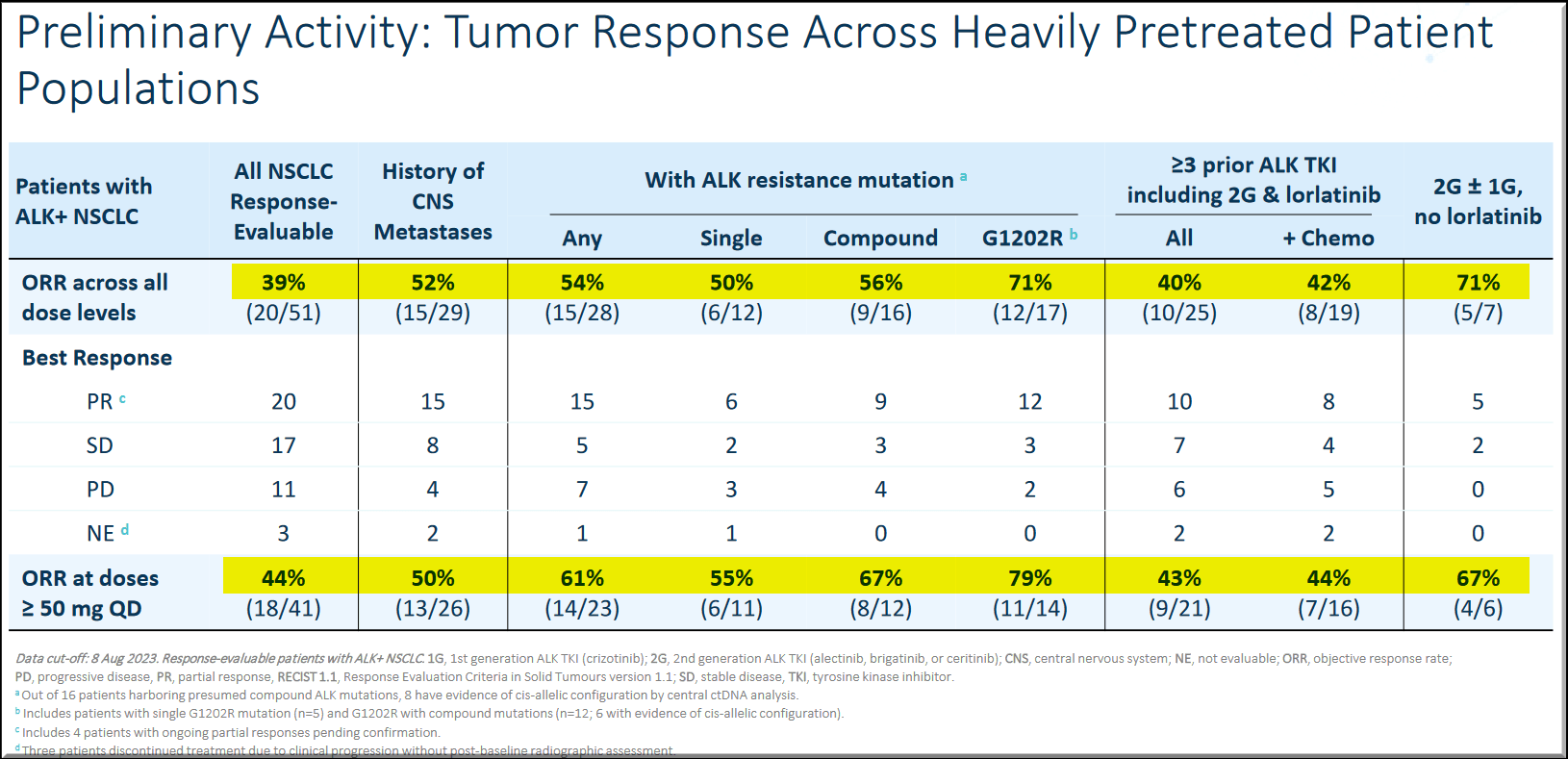
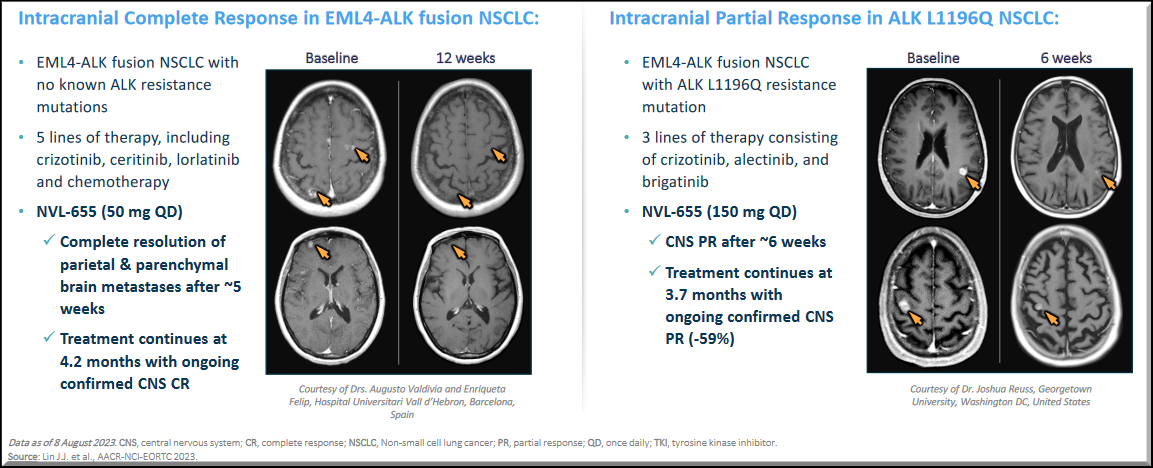
In the Phase 1 dose-escalation portion of the ALKOVE-1 trial, NVL-655 showed a favorable safety profile consistent with an ALK-selective, TRK-sparing design, exceeding the targeted CNS efficacy thresholds for ALK mutations. Objective responses were observed in heavily pre-treated patients, including those with ALK mutations and CNS metastasis.
The NVL-655 treated brain scans have shown that the experimental drug-induced intracranial responses in patients with ALK+ NSCLC.
NVL-655 offers an opportunity to be a potential best-in-class drug to advance the treatment paradigm and deliver durable responses.
The Phase 2 portion of the ALKOVE-1 trial began enrolling patients in February 2024, aiming to evaluate NVL-655's efficacy in TKI pre-treated and TKI-naïve ALK-positive NSCLC patients. The primary endpoint is the ORR, and secondary endpoints include efficacy measures and intracranial activity. Updated Phase 1/2 data is expected in the second half of 2024.
NVL-330 for HER2-Mutant NSCLC
Mutations in the human epidermal growth factor receptor 2 (HER2 or ERBB2) are found in up to 4% of metastatic NSCLC cases. In-frame deletions, insertions, or duplications in exon 20 (HER2ex20) account for 90% of these cases.
About 20% of patients with HER2-mutant NSCLC are also present with brain metastases, a figure that increases with treatment. Current therapeutic options targeting these mutations are limited.
NVL-330 is a brain-penetrant, HER2-selective TKI designed to treat HER2ex20 mutations-driven tumors, minimize off-target inhibition of wild-type EGFR, and treat brain metastases.
Clinical Insights NVL-330
Preclinical data showed NVL-330's efficacy in inhibiting HER2ex20, brain penetration, and selectivity over wild-type EGFR.
A Phase 1 clinical trial is being planned for later in 2024.
Financial Position & Risks
As of March 2024, Nuvalent had $692 million in cash and equivalents with no long-term debt. These resources are expected to fund the company's operations into 2027, including Phase 3 trials for one to two of its candidates.
While Nuvalent's candidates show promise, they are still in the early stages. Biotechnology valuations are data-dependent, and the limited pipeline of young biotech companies without clinical products concentrates risk. The sector is known for higher investment risk and volatility.
Conclusion
Nuvalent's pipeline includes two promising precision-targeted oncology treatment candidates, which address the challenges encountered by current treatments. With expertise in molecular structural design, the company selectively identifies potential candidates and can extend this platform approach to other indications. Both NVL-520 and NVL-655 have potential best-in-class profiles, supported by early-stage data. Nuvalent's OnTarget 2026 plan aims for potential first approval in 2026.
Interim Phase 1/2 data for both the ROS1 and ALK programs will be the next milestone that can affect valuation. The pivotal Phase 2 data next year if successful could significantly boost the company valuation, and possibly elicit acquisition interest.
We believe Nuvalent represents a speculative investment opportunity for investors based on its innovative pipeline of novel wholly-owned candidates, the platform approach, and market needs. We anticipate that favorable upcoming Phase 1/2 updated data, positive Phase 2 data next year, and a more favorable lower-yield macro environment for biotech stocks, can contribute to a stock price advance of 50% to 100% from current levels over the next six to twelve months. At the same time, the prominent risk for the stock is mediocre Phase 2 data that misses the endpoints for the two drug candidates.
Nuvalent is part of the Prudent Biotech model portfolio. The position can be closed and our opinion can change without public notification. Investors should conduct due diligence and understand the risks in speculative biotechnology stocks.
______________________
The article was first published exclusively on Seeking Alpha