Company Overview
Cidara Therapeutics (NASDAQ:CDTX) is a biotechnology company developing novel immunotherapies to prevent and treat infectious diseases and cancer. Through its proprietary Cloudbreak platform, the company is pioneering drug–Fc conjugates (DFCs) - “single-molecule cocktails” that combine small-molecule and peptide drugs with a human antibody fragment (Fc) to harness immune mechanisms for targeted, durable efficacy.
Cidara’s lead candidate, CD388, is a long-acting antiviral prophylactic designed for universal prevention of seasonal and pandemic influenza through a single dose per season. CD388 represents the company’s primary value driver and is the most advanced candidate of its Cloudbreak platform.
Unique Mechanism of Action: Redefining Flu Prevention
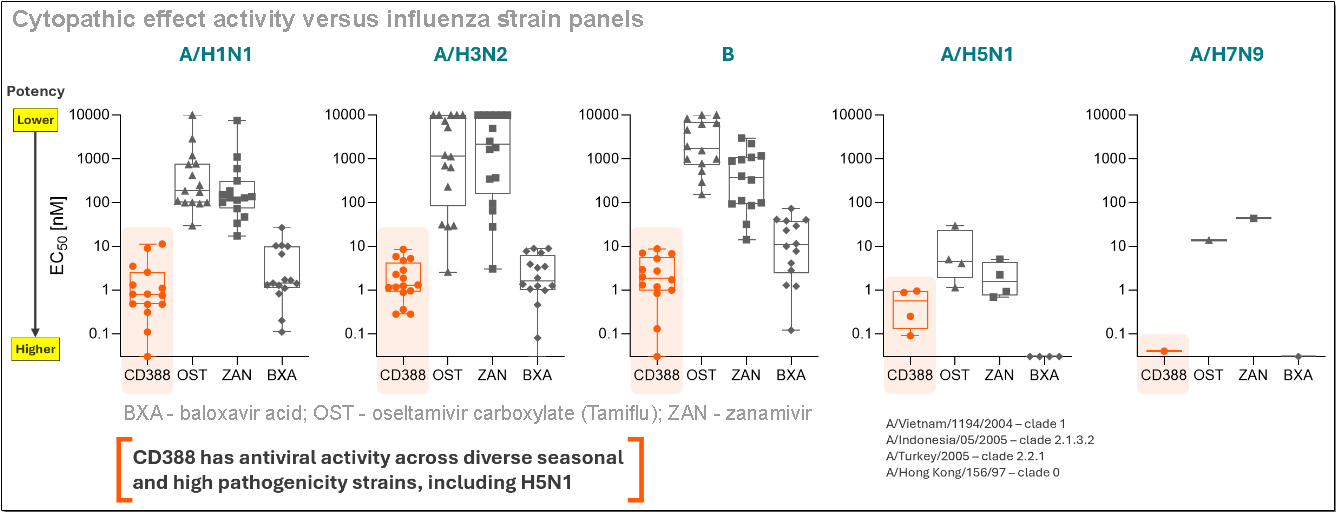
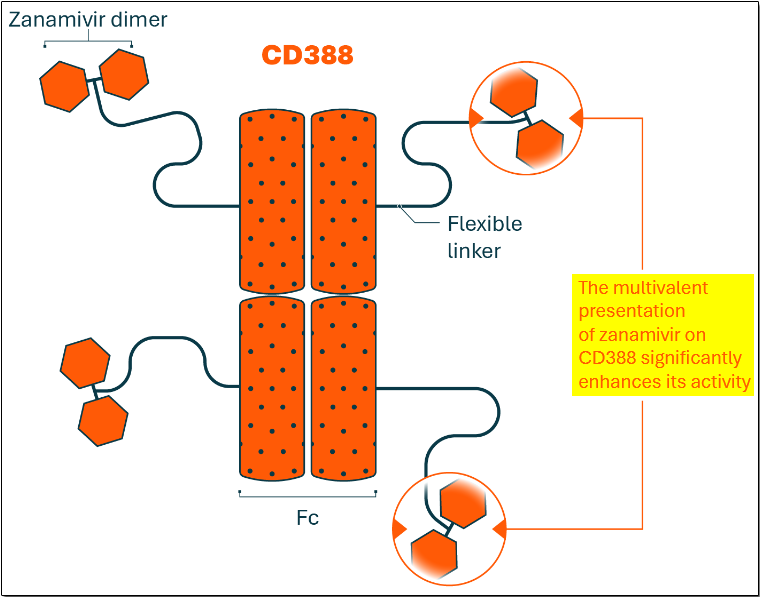
CD388 links zanamivir (the active ingredient in GlaxoSmithKline’s Relenza) to an antibody Fc fragment engineered for extended half-life. A single dose of this combination is designed to provide broad and durable protection against all influenza A and B strains, including highly pathogenic variants such as H5N1 (bird flu), with a single dose.
The drug arrays multiple copies of zanamivir on the antibody fragment, which significantly enhances its activity.
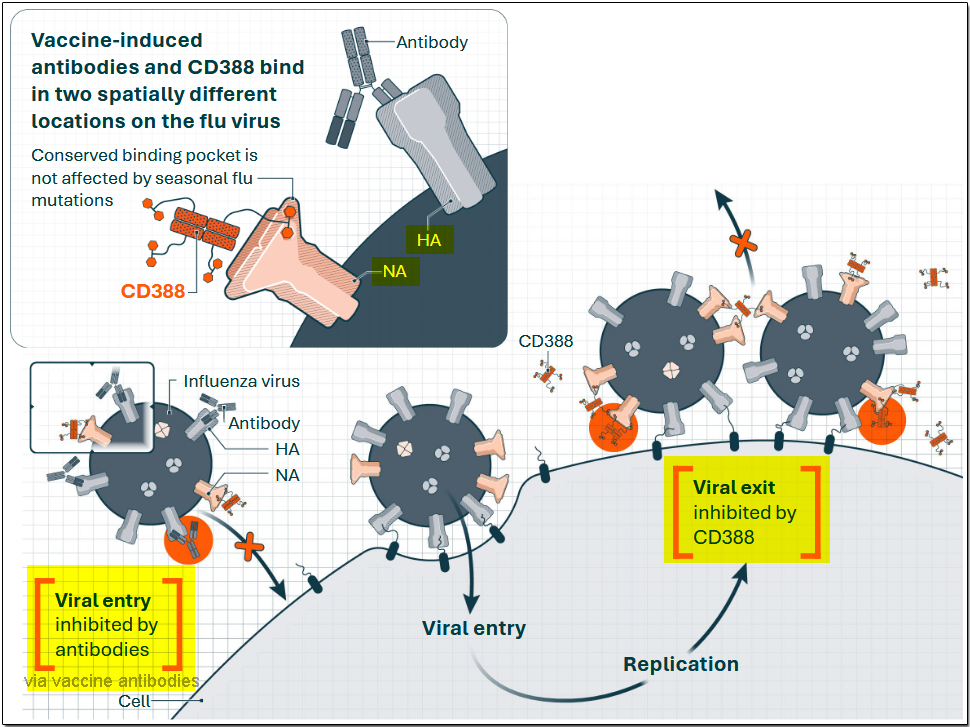
Unlike vaccines that stimulate an immune response to specific flu strains, CD388 works by directly inhibiting viral replication, preventing the virus from spreading once it enters the body. The slide below shows the approaches of the vaccine and CD388 to overcoming the flu virus.
In the above slide, HA stands for hemagglutinin and NA stands for neuraminidase. These are major surface glycoproteins on a virus that work together to infect a host cell and spread the virus. Both of them are essential for virulence, as determined by a combination of factors, including the virus's ability to evade the host's immune system and to replicate efficiently to spread throughout the body and damage cells. Vaccine-induced antibodies target and bind to the hemagglutinin, or HA, site on the flu virus in an effort to prevent it from infecting the host cell. In contrast, CD388 antiviral binds to the neuraminidase site to prevent the virus from replicating and spreading itself. CD388 does not interfere with the actions of vaccine antibodies, as each of them binds to spatially different locations. Thus, they can work in a complementary manner to create a more robust flu shield, attacking the virus at both its entry and exit points.
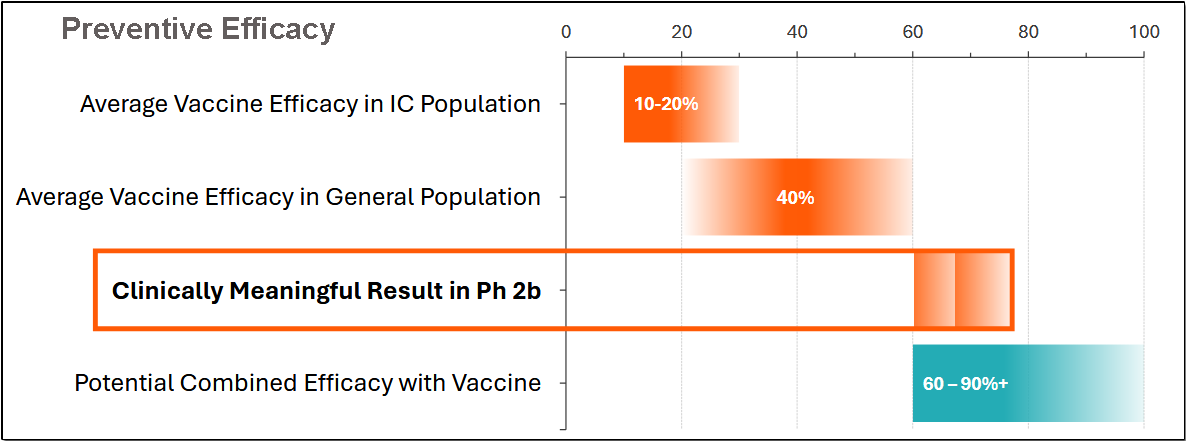
It should be noted that trials have determined that CD388 efficacy is not impacted by patient vaccination status.
CD388’s activity does not rely on an immune response, which makes it particularly well-suited for elderly and immunocompromised patients who are hyporesponsive to vaccines or cannot receive them due to contraindications.
Clinical Progress and Key Developments
Phase 2b NAVIGATE Trial
CD388 has been granted both Fast Track and Breakthrough Therapy Designations by the FDA for influenza prevention in adults and adolescents at high risk for flu complications.
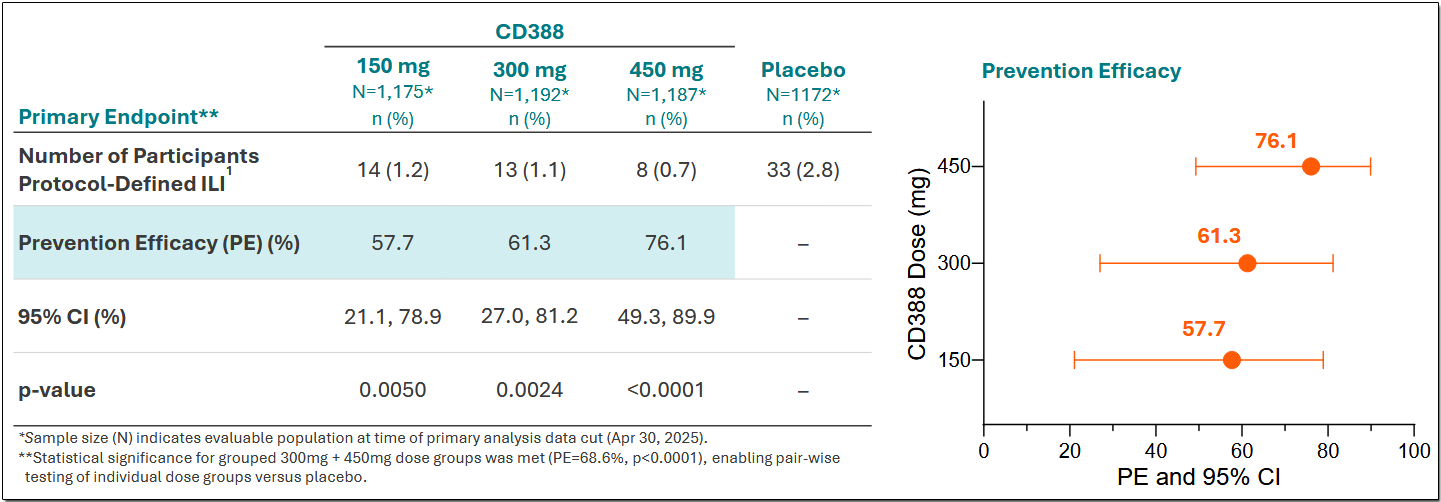
In June 2025, Cidara announced positive results from the Phase 2b NAVIGATE trial, which evaluated CD388 in healthy, unvaccinated adults (ages 18–64).
FDA End-of-Phase 2 Meeting Outcome and Phase 3 Framework
Following a successful End-of-Phase 2 meeting in September 2025, the FDA approved a streamlined path to registration that was very favorable for the company, as summarized below.
Following the FDA feedback, the company was able to accelerate its trial plans to begin enrollment from September 2025 itself in the Northern Hemisphere with continuation into the spring of 2026 in the Southern Hemisphere. This was a six-month acceleration from the prior plan, which started with the Southern Hemisphere, followed by the Northern Hemisphere in Fall 2026.
The Phase 3 ANCHOR study design will have participants randomized in a 1:1 ratio to receive either a 450-milligram dose of CD388 or a placebo. The primary endpoint will be based on laboratory-confirmed influenza, a body temperature of 37.2°C (99°F), and new or worsening of either two respiratory symptoms (cough, sore throat, or nasal congestion) or one respiratory symptom and one new systemic symptom (headache, fatigue, feeling feverish, or body aches).
Cidara began dosing participants in the Phase 3 study in September 2025. The trial will include an interim analysis after the Northern Hemisphere flu season to confirm powering assumptions and determine enrollment for the next stage.
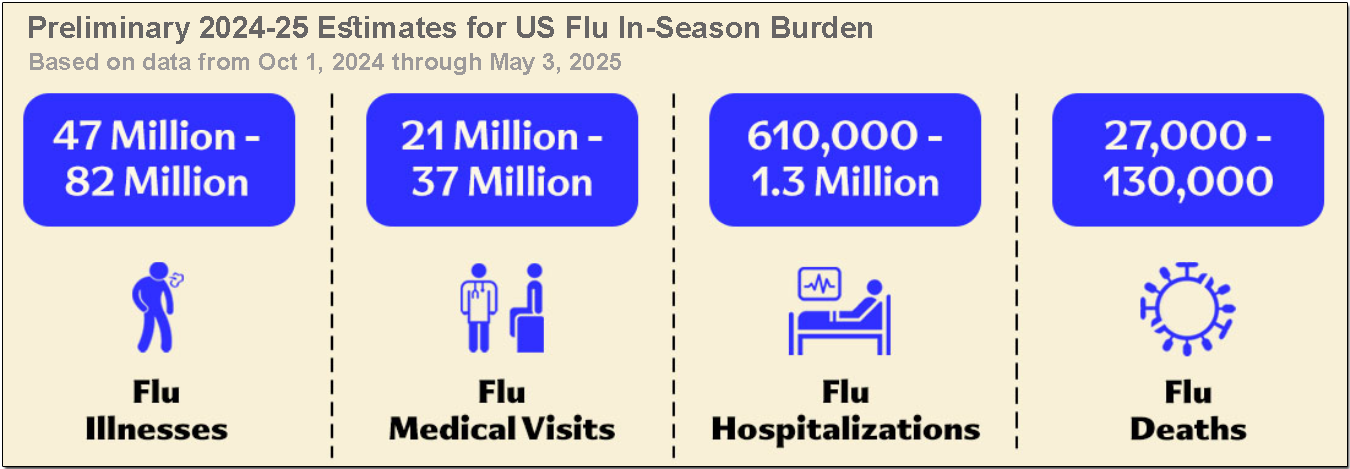
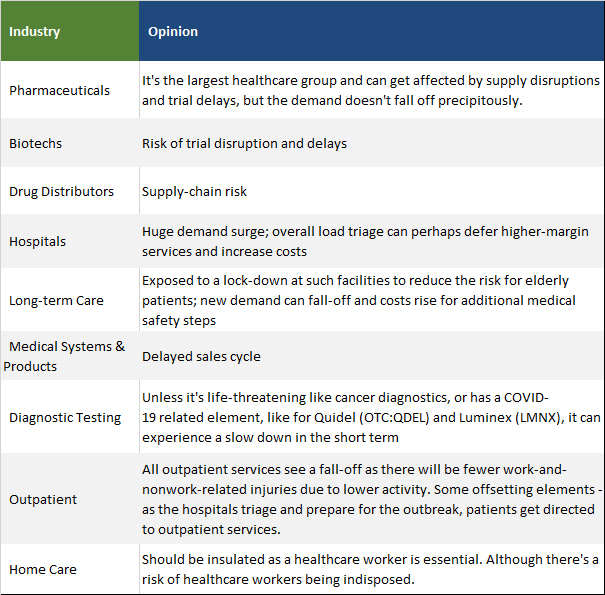
This is due to various reasons, which include:
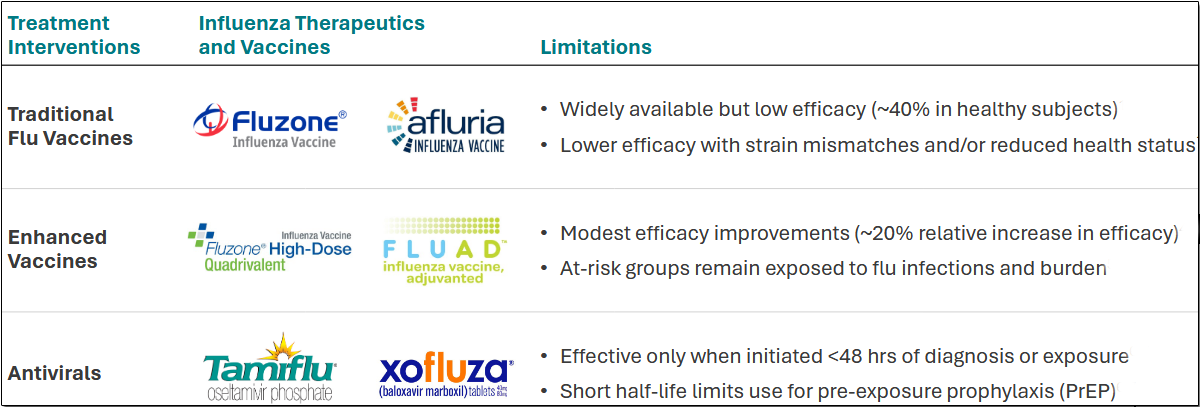
The slide below shows the limitations of current influenza interventions.
CD388 can address the limitations and shortcomings of the present standard of care, including efficacy, time-to-treatment, and an unfavorable policy regime.
Cidara's CD388 provides:
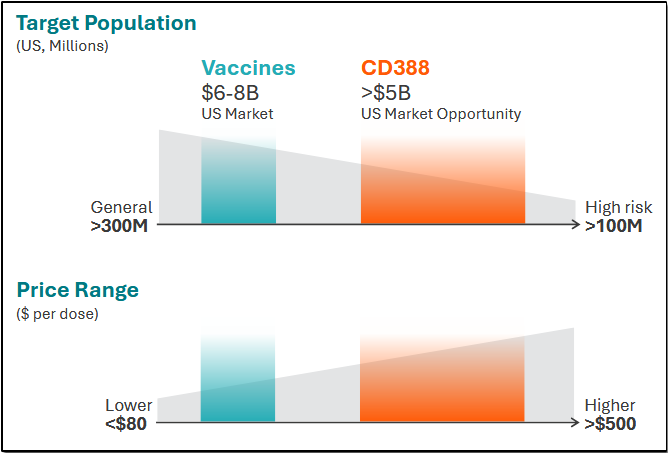
With a U.S. eligible pool of greater than 100 million, the uniquely positioned CD388 is addressing a market size that can exceed $5 billion.
Risks
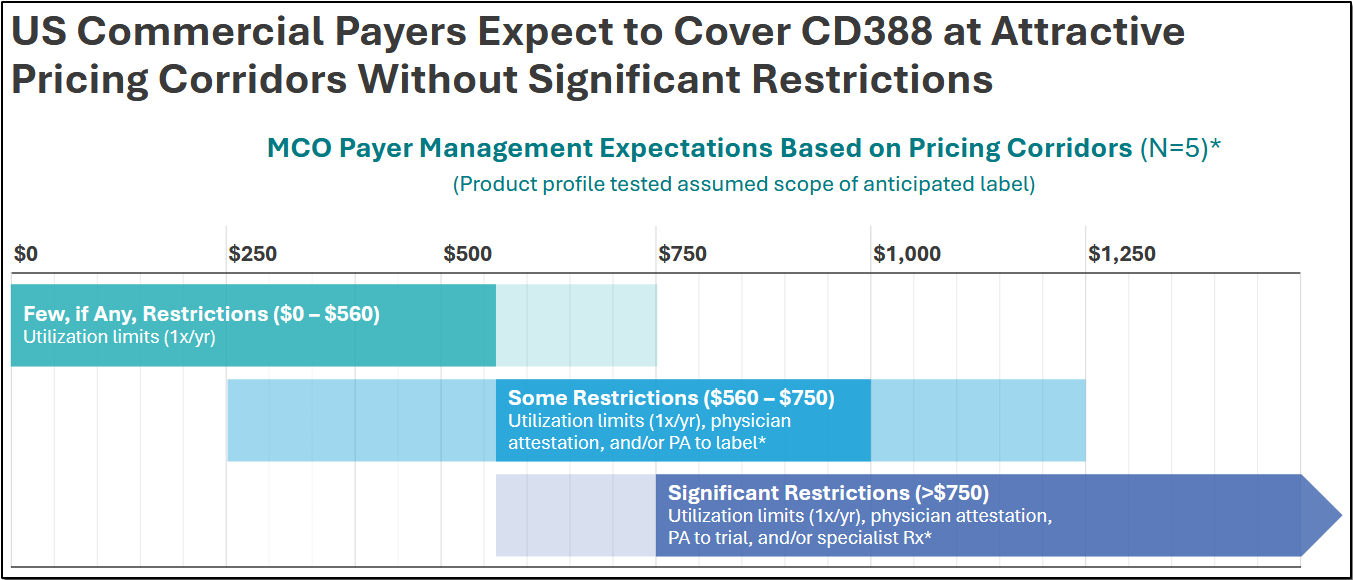
The Phase 3 trial has to deliver data that meets the endpoints and confirms the Phase 2 efficacy and safety. While the Phase 3 design and the earlier trial results significantly increase the likelihood of a favorable outcome, there is always an element of risk that the data may not be as expected. Any delay in trial results or submission could impact timelines. Transitioning from trial-scale to commercial production can pose execution challenges. Sufficient insurance payer coverage and pricing rates are also risks that will determine access to CD388. The company expects to encounter little resistance to its pricing based on the disease burden.
Conclusion
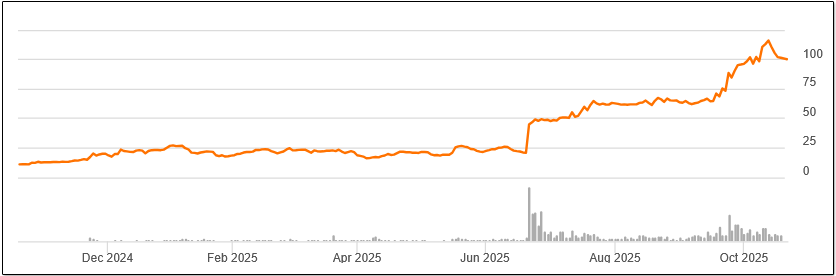
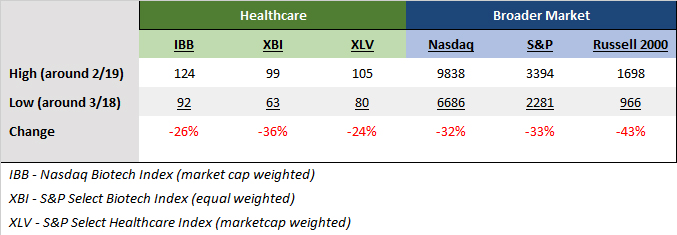
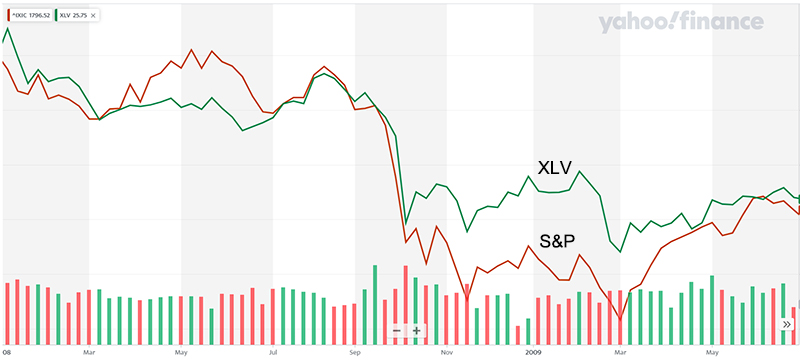
Biotechnology is in a favorable environment marked by a lower 10-year US Treasury yield, a new Fed easing cycle, positive data outcomes, and renewed investor appetite. The biotech indices are up double digits year-to-date, and momentum is returning to innovation-driven names. As of October 17, the NYSE Biotechnology Index was up 17% for the year, and the S&P Select Index has risen nearly 20%. The Prudent Biotech model portfolio is up over 40% for the same period.
Cidara’s CD388 represents one of the most compelling prophylactic antivirals in development - a potential new standard of care for influenza prevention. Its once-yearly dosing, universal strain coverage, independence from immune response, and addressing an unmet need for a more effective flu prophylactic create a differentiated and defensible market position.
If the Phase 3 ANCHOR trial succeeds in 2026, Cidara could be positioned for blockbuster status and platform validation, potentially transforming the company’s valuation trajectory and, in our opinion, catapulting the stock to the $175 to $200 range over the next 12 months.
Cidara Therapeutics is currently held in the Prudent Biotech model portfolio. Positions and opinions are subject to change without notice. Investors should conduct their due diligence and recognize that biotechnology stocks carry elevated risk.
More details on CD388 trial data can be found in the corporate presentation.
(The article was first published on Seeking Alpha.)