The FDA's decision to approve Biogen's Aducanumab, now Aduhelm, will stand out as one of its biggest decisions in recent memory due to its profound implications. Besides being intensely controversial, it has left bold footprints on the regulatory pathway for others now to follow.
In a recent article, Biotechs Ready to Roar, it was pointed out how the FDA's use of an accelerated approval track to accept a positive surrogate endpoint for Aduhelm, even when the primary endpoint was not met, as the basis for an accelerated approval has created a more visible path for other neurological companies to pursue. This FDA approval was for a significant unmet need for treatment in Alzheimer's disease. The accelerated approval requires a confirmation study and in the case of Aduhelm, Biogen was provided with up to nine years by FDA to complete the study.
The decision sets an important precedent for neurological treatments for high unmet needs like Alzheimer's, Parkinson's, and similar diseases. First, the decision rejuvenates treatments using the beta-amyloid hypothesis as the primary endpoint of an improvement in the disease may not be required, prior to accelerated approval. Trials based on the hypothesis over the last two decades have failed to show a clear signal of an improvement in the disease, the principal reason why the approach was teetering prior to Aduhelm's approval. Second, the approval boosts different treatment approaches as an improvement on the lower bar of performance set by Aduhelm can have the potential to create a blockbuster treatment.
There is a risk that the FDA reconsiders the precedent-setting guideline and is reluctant to use the fast-track due to the controversy. The FDA's precedent will be put to test as Eli Lilly (LLY) files for a Biologics License Application (BLA) based on Phase 2 data for its drug Donanemab, which is similar to Aduhelm.
Nonetheless, Aduhelm's approval can be considered a significant regulatory derisking event that has turned a pool of promising potential treatments into highly valued assets. In April's article, 3 Neurology Stocks, we had discussed three of the many attractive small and midcap companies. Today, we discuss one of these in greater detail.
Prothena
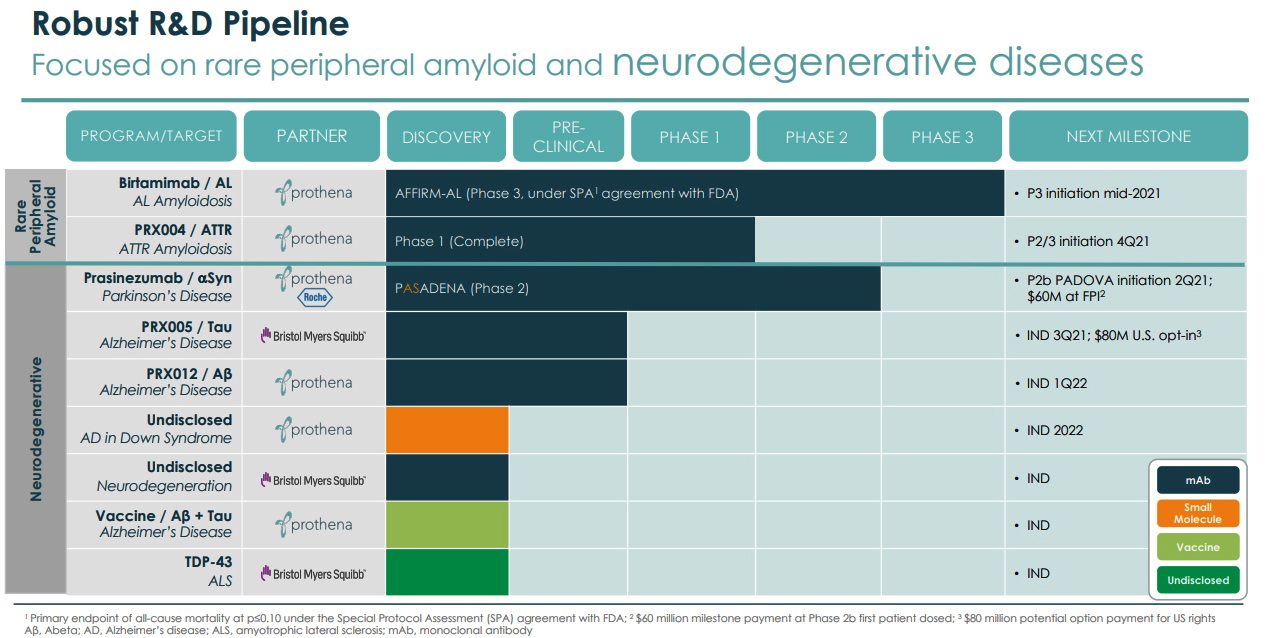
Prothena (PRTA) is a 2012 spin-off from the Irish company Elan Corporation. It has a pipeline of fully-owned and partnered programs that are focused on peripheral amyloid and neurodegenerative diseases, leveraging its expertise in protein dysregulation and understanding the biology of misfolded proteins and neurological dysfunction. The recent excitement for Prothena has been generated because of its expertise in clearing amyloid plaque, a strength that becomes more evident as one goes through the pipeline. The correlation to Biogen's success with Aduhelm is clear.
Prothena's pipeline covers neurodegenerative diseases and peripheral amyloid.
Parkinson's Disease
Prothena's program, Prasinezumab, in early Parkinson's disease is the first potentially disease-modifying antibody to demonstrate signals of efficacy on multiple pre-specified clinical endpoints in patients with early Parkinson's disease. It is the first anti-alpha-synuclein antibody to advance into late-stage development.
Parkinson's is a progressive and degenerative disorder that affects the entire central nervous system (CNS). Over 10 million suffer from this movement disorder with nearly 1 million in the US. Each year, 60,000 people in the US are diagnosed with Parkinson's, as per the Parkinson's Foundation. While a diagnosis of Parkinson's relies on the motor symptoms typically associated with it, non-motor symptoms related to the disease may present themselves many years earlier.
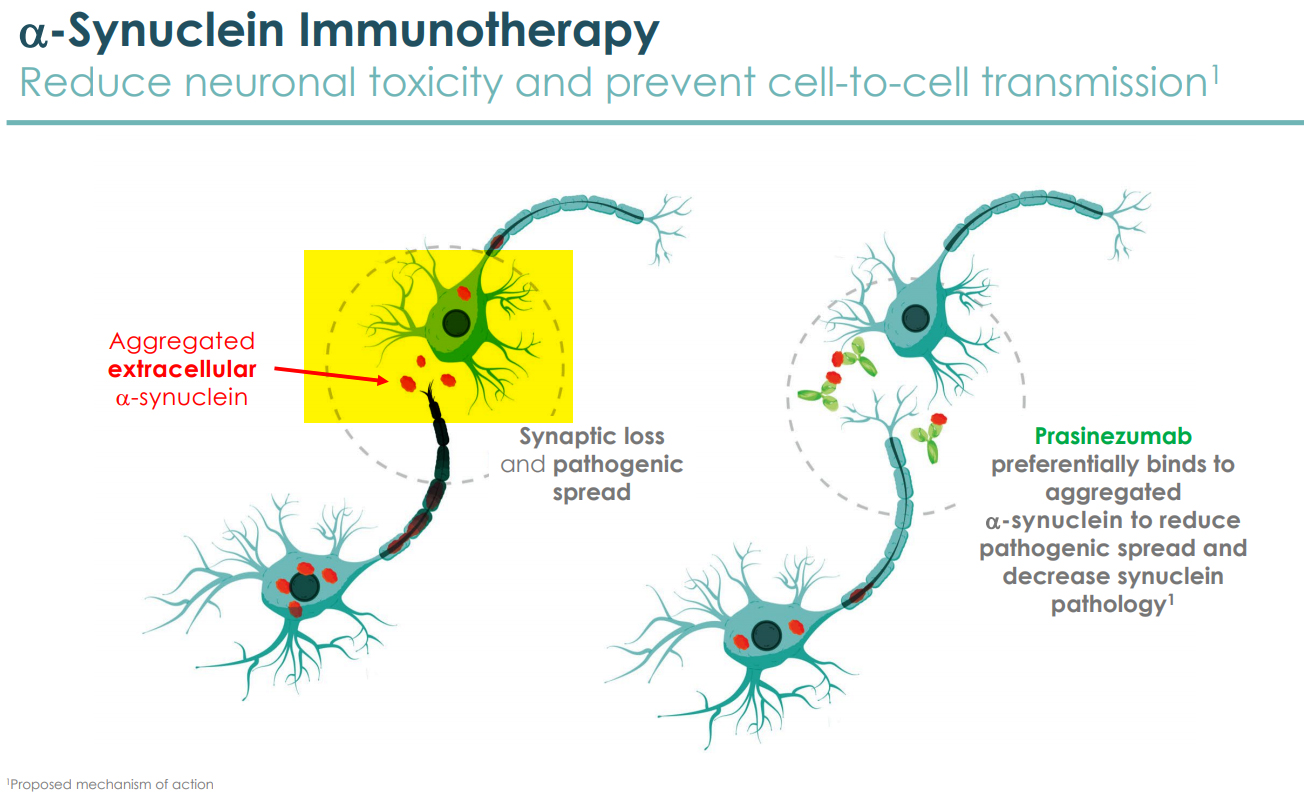
Parkinson's disease is characterized by the accumulation of the alpha-synuclein (α-synuclein) protein in the CNS and peripheral nervous system. There is genetic evidence of the causal role played by α-synuclein in Parkinson's disease.
Alpha-synuclein is a protein found in neural tissue. Mutations to the protein sequence can cause it to aggregate and spread from cell to cell. There is a growing body of evidence that the disease-causing α-synuclein can be transmitted from neuron-to-neuron resulting in the loss of neuronal activity and the spreading of neuronal death. Such neurodegeneration can be diminished by targeting the errant forms of α-synuclein protein. That is the focus of Prothena's antibody Prasinezumab, a therapeutic that targets α-synuclein by blocking the cell-to-cell transmission of the errant or pathogenic aggregates of α-synuclein, thereby slowing the progression of the disease and the clinical decline of patients. Existing treatments are unable to achieve that and are mostly symptomatic.
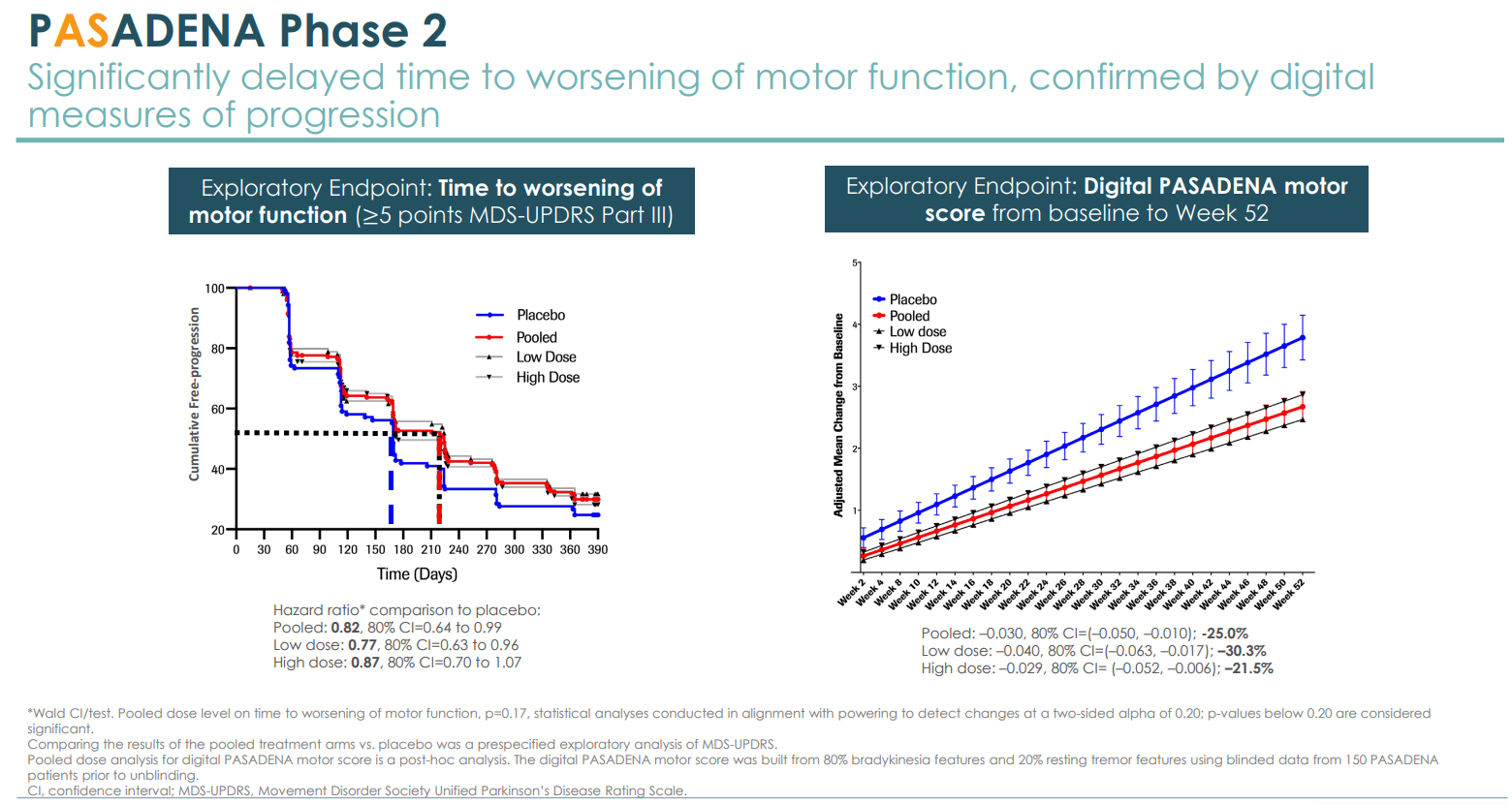
Prasinezumab has stood out for its strong results so far. In a Phase 2 study, Prasinezumab significantly reduced the decline in motor function on the Parkinson's Disease Rating Scale by 35% versus the placebo, after a year of treatment.
Partnered globally with the Swiss pharmaceutical giant Roche on the program, the two companies are advancing the investigative treatment into Phase 2b study. Prothena has already earned a $60 million clinical milestone payment upon the dosing of the first patient last month. The licensing agreement can allow Prothena to receive up to an aggregate of $600 million in upfront and milestone payments from Roche, of which $135 million has already been received, and a 70/30 split between Roche and Prothena, respectively, for all costs and profits.
Alzheimer's Disease
Prothena has two preclinical programs in Alzheimer's Disease. These include the PRX005 and PRX012. Although still in preclinical, the speed with which a promising program can now potentially advance and achieve breakthrough therapy for Alzheimer's and a fast-track approval in a much shorter time frame was underscored by the FDA's nod for Aduhelm. With 6 million people affected by the fatal Alzheimer's disease, it has become the most common neurodegenerative disorder in the country, with a high unmet need for disease-modifying treatments.
To get a quick understanding of Alzheimer's, one has to understand what are its characteristics and the key elements of a neuron. An Alzheimer's affected brain has five key characteristics - amyloid plaques, neurofibrillary tangles, chronic inflammation, vascular issues, and loss of neuronal connections leading to cell deaths. A brain cell or neuron has three basic parts - the cell body which contains the nucleus, the dendrites which extend out of the cell body and are receivers of information from other neurons, and the axon which is the thicker cable-like structure at the end of the cell body, opposite from the dendrites, that transmits messages to other neurons.
The beta-amyloid (β-amyloid) protein is widely known to be associated with Alzheimer's disease by creating amyloid plaque and interfering with communication between neurons.
Tau is another protein whose abnormal accumulation inside the neuron cells results in neurofibrillary tangles that are implicated in Alzheimer's disease. Neurons, in part, are internally supported by structures called microtubules, that transport nutrients from the cell body to the dendrites and the axon, and aid in communication. In a healthy neuron, the tau protein binds to and stabilizes the microtubules. However, during Alzheimer's disease, the tau protein detaches from the microtubules and begins to bind with each other inside a neuron in thread-like structures that join to form tangles.
While the β-amyloid plaque builds up between the nerve cells breaking down the cell-to-cell communication, the twisted tau fibrils work internally within the cell and end up blocking the transport structure within the neuron, eventually starving it and diminishing the ability for synaptic communication between neurons. The pathogenic tau fibrils propagate further by easily transmitting between cells, diminishing or destroying healthy such neuron cells. It has been observed that after a tipping point is reached in the accumulation of β-amyloid, tau spreads rapidly to all regions of an Alzheimer's affected brain, correlating to the worsening of the disease.
Prothena's PRX012 program targets the Amyloid Beta, while the PRX005 targets the Tau.
Aß - PRX012
The PRX012 antibody targets the Amyloid Beta (Aβ or Abeta), which denotes peptides of 36-43 amino acids that are the main component of the amyloid plaques found in Alzheimer's disease. The aggregation or clumping of Aβ into small soluble oligomers, protofilaments, and fibrils eventually leads to plaque deposits in human brains.
Studies of monoclonal antibodies have demonstrated that targeting key epitopes within the N-terminus of Aß reduces the amyloid plaque burden which is associated with the slowing of Alzheimer's disease. Biogen's Aduhelm is a similar type of antibody directed at the N-terminus, though no clear signal was present in the Phase 3 study with regards to slowing the decline in Alzheimer's disease. However, a precedent has been set. Reducing amyloid plaque is sufficient evidence for FDA approval using the accelerated approval pathway even with an unproven inference that such lowering can potentially have benefits in diminishing symptoms for Alzheimer's disease.
Prothena has developed potent Aβ antibodies, leveraging its epitope scanning platform, that it believes are the same or better in efficacy and with a more convenient subcutaneous administration rather than infusions for most other approved (Aduhelm) and investigational drugs.
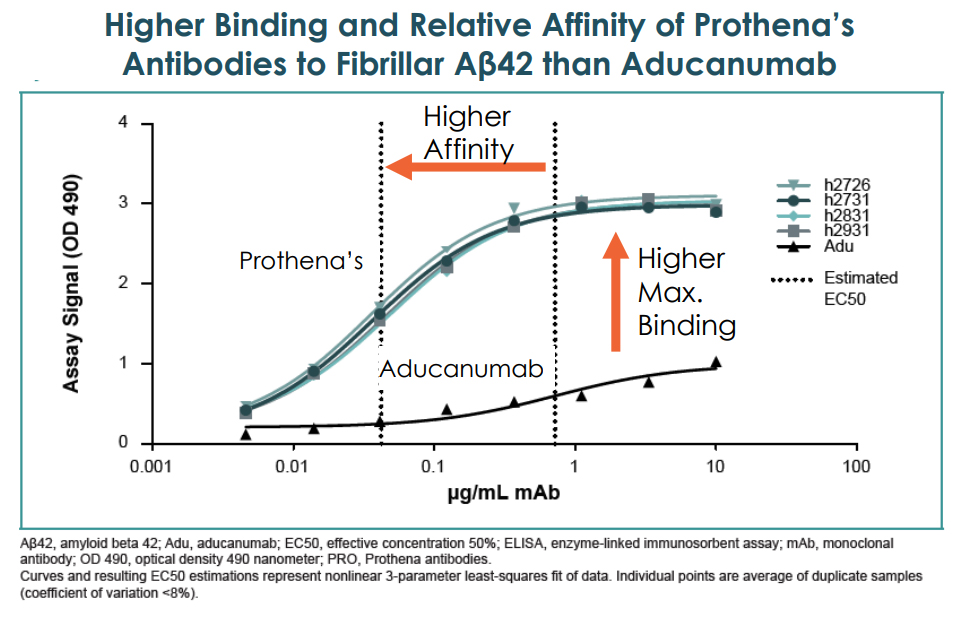
Based on pre-clinical studies, PRX012 has a higher binding strength to amyloid plaque than Aduhelm, which is intravenously administered. In addition, unlike Aduhelm which mostly targets insoluble plaques, PRX012 also neutralizes soluble, toxic oligomeric Aß species. (Biogen's Japanese partner Eisai's drug, BAN2401, will target soluble aggregate or protofibrils).
The PRX012 has an 11-fold greater affinity to fibrillar Aβ than Aduhelm and shows a greater ability to recognize Aβ pathology, which would mean a more extensive area of coverage for plaque binding, even at lower antibody concentrations. These powerful characteristics of higher efficacy and easier dosage administration create a very promising early-stage therapeutic.
Prothena owns the rights to PRX012 and is expected to file an Investigational New Drug Application (IND) in the first quarter of 2022, though with intense interest following Aduhelm's approval it is quite likely the Company will look for ways to accelerate the timetable.
In an earlier article this month, Biotechs Ready to Roar, we had discussed what is good for Biogen's Aduhelm, is now going to be great for others. In particular, it was noted that Aduhelm will face a material risk of being outperformed soon.
... with the bar for surpassing Aduhelm's performance now set quite low, there is also a very good likelihood of relatively better treatments emerging in the next 3 years or sooner.
Investigational therapeutics like PRX012 can potentially be the ones to materially outperform Aduhelm.
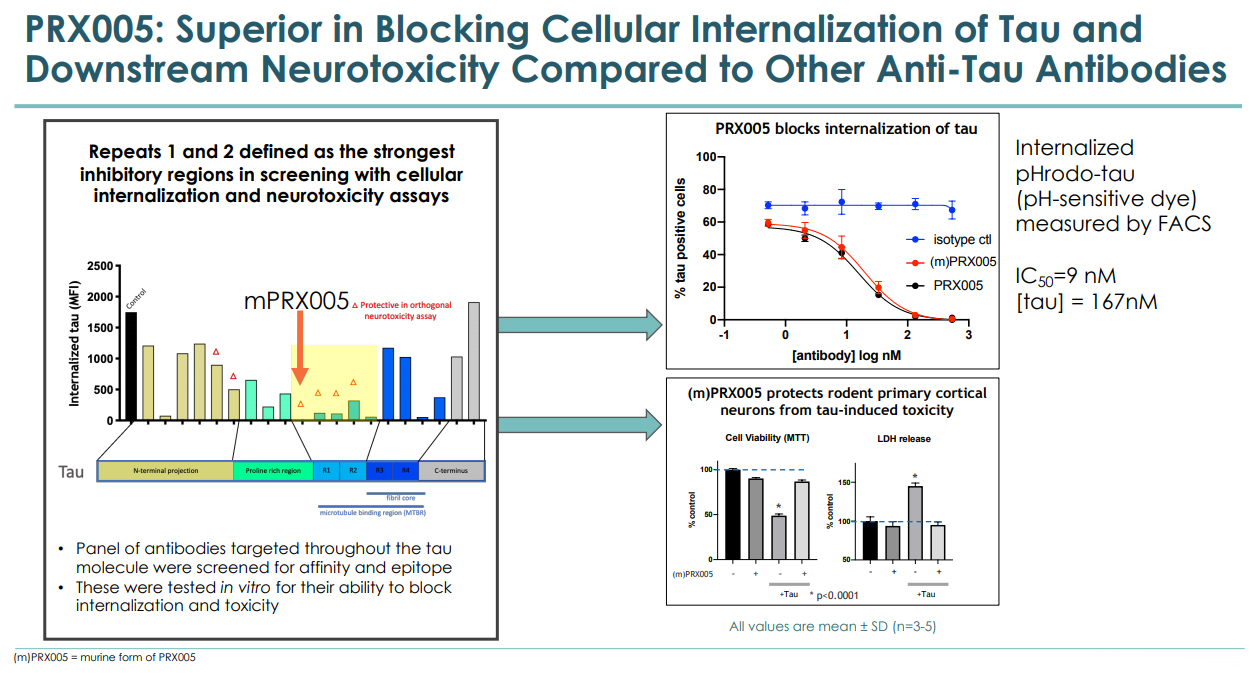
Tau - PRX005
The PRX005 is an antibody that targets pathogenic tau, which is implicated in Alzheimer's disease and multiple other neurological diseases.
The PRX005 is being developed to target the epitopes within the microtubule-binding region (MTBR) of tau. Other treatments are being developed as well targeting the epitopes on pathogenic tau. Prothena's ability to screen and discover optimal epitomes is a distinguishing ability of its scientific platform and one that the company believes has allowed it to identify a key epitope within the microtubule region. In preclinical studies, PRX005 demonstrated blocking of tau internalization and slowing the pathological progression in a tau transgenic mouse model by targeting the specific epitope.
PRX-005 is promising though still in an early stage as it tackles a highly complicated state of pathogenic tau. The failure earlier this month of Biogen's investigational treatment Gosuranemab (BIIB092), an investigational anti-tau antibody, in a Phase 2 study is a reminder of the risks. To that extent, last week's support of PRX005 by a major pharmaceutical is encouraging, as discussed below.
Bristol Myers Squibb Agreement
When it acquired Celgene, Bristol Myers Squibb (BMY) ended up inheriting a $50 million equity stake in Prothena along with the option to exclusively license three programs.
Prothena is expected to file an IND for PRX005 in the third quarter of this year. At that time, Bristol Myers had to decide on exercising its option for exclusive US rights. However, Bristol Myers didn't wait that long and last week acquired the option for US rights to PRX005, the best-in-class anti-tau antibody, triggering an $80 million payment to Prothena. The continued collaboration is an encouraging sign.
Once Phase 1 is completed, Bristol Myers has the remaining option for global exclusivity for another $55 million payment, after which it will lead and fund the entire development of PRX005 till commercialization. The total payments from Bristol Myers for the three programs can reach up to $2.2 billion, besides tiered royalties.
In addition to Aß (PRX012) and tau (PRX005), Prothena has 4 other early-stage programs in neurodegenerative diseases, including two such programs in partnership with Bristol Myers.
Peripheral Amyloid Rare Diseases
Prothena's has two fairly advanced programs in peripheral amyloid. The AL Amyloidosis investigational therapeutic, which is the most advanced in Prothena's pipeline, is expected to begin Phase 3 in the third quarter. The other program in ATTR Amyloidosis will begin Phase 2 later this year.
AL Amyloidosis - Birtamimab
Prothena's investigational drug is a potential treatment for a rare and orphan disease, Amyloid Light chain (AL) amyloidosis. This disease is a progressive and fatal disease that causes organ dysfunction and eventual failure due to immunoglobulin light chain proteins, produced by plasma cells, misfolding, aggregating, and depositing as amyloid clusters in vital organs, a similar concept to what occurs with misfolded proteins and amyloid plaque in brain cells.
Birtamimab, which has received fast track designation by the FDA, has a depleting mechanism of action that targets the misfolded light chain proteins to clear away the amyloid plaque. Presently, the only therapeutic available has not shown any survival benefit and focuses more on reducing new protein production and not directly clears the amyloid deposits in organs. Removing such amyloid aggregates is where Prothena has shown expertise. The drug neutralizes the soluble aggregates in misfolded proteins and spurs clearance of amyloid. It is the only investigational treatment to have shown a survival benefit in a placebo-controlled study with 74% of the patients alive at nine-month compared to 49% for the control group.
In February 2021, after discussions with the FDA, Prothena announced the program is going to advance into confirmatory Phase 3 trials in the second half of this year with a primary endpoint of all-cause mortality.
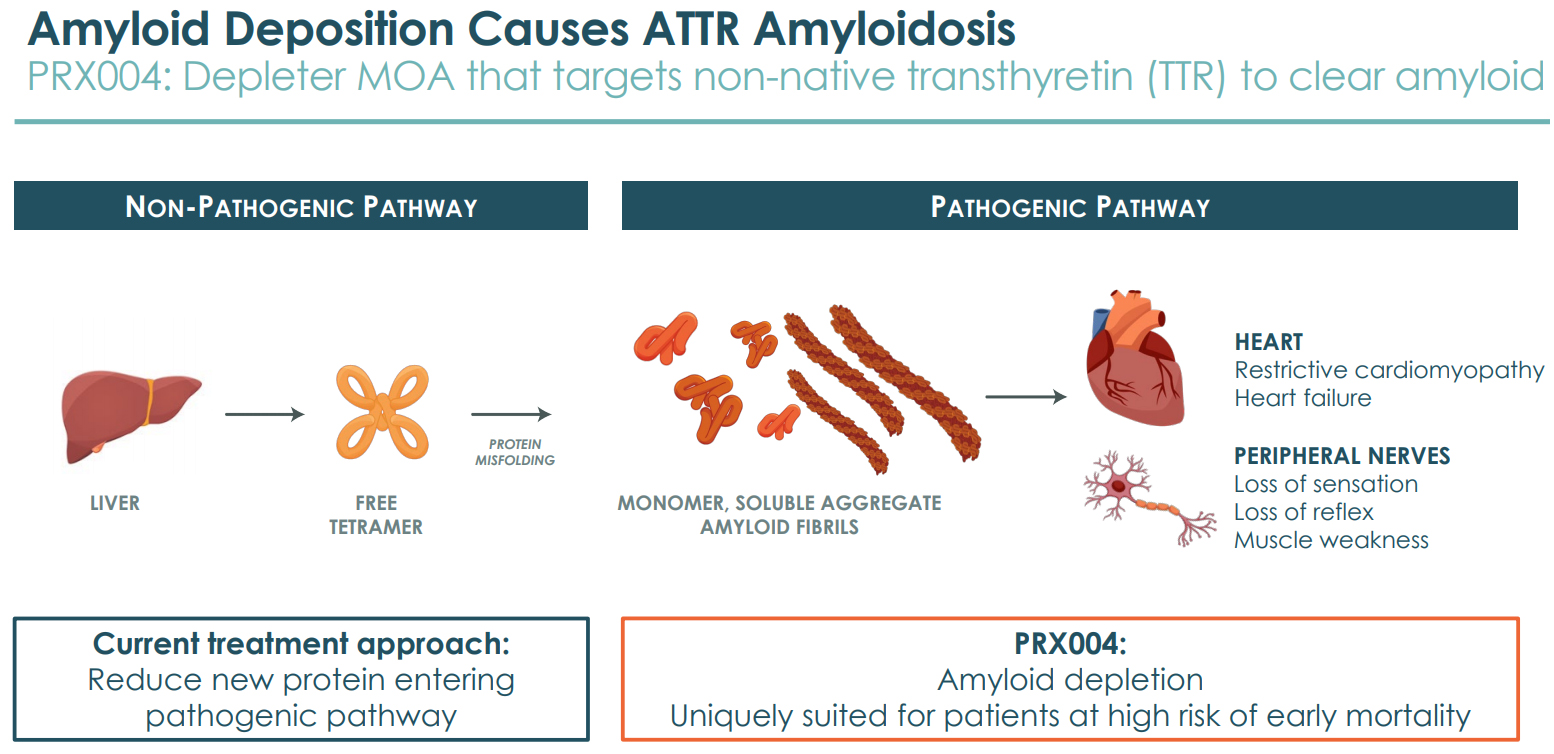
ATTR Amyloidosis - PRX004
ATTR amyloidosis is a rare, progressive, and fatal disease characterized by the buildup of amyloid deposits composed of misfolded forms of mutant or non-native Transthyretin protein (amyloid) in vital organs, most commonly the heart and the peripheral nerves in the nervous system. The disease can be hereditary (hATTR) when caused by a Transthyretin or TTR protein gene mutation, or wild-type (wtATTR) which can occur irregularly. It is estimated that between 400,000 to 1.4 million patients suffer from ATTR-cardiomyopathy due to such deposits, of which between 130,000 and 490,000 patients are estimated to be moderate-to-advanced.
PRX004 is a monoclonal antibody, which has been designed with a differentiated mechanism of action to target and clear the non-native TTR aggregates (amyloid) without affecting the native form of TTR protein. The deposits are believed to contribute to organ dysfunction and failure. PRX004 is a mutant TTR depleter and differentiates from existing therapies by not just preventing new formation, but also clearing amyloid deposition as well.
In the Phase 1 study results announced in Dec 2020, PRX004 demonstrated a slowing of neuropathy progression, using the depleter mechanism, for all 7 patients, and an improvement in 3 patients at 9 months.
Based on these Phase 1 results, Prothena is advancing the program into a Phase 2/3 study in moderate-to-advanced ATTR-cardiomyopathy patients. The study is expected to initiate in the fourth quarter of this year. The program rights are entirely owned by Prothena.
Prothena's Broad Expertise in Clearing Amyloid
Reviewing Prothena's pipeline and its successes so far, what clearly stands out is its broad and deep expertise in targeting and removing amyloid plaque and toxic aggregates in different regions of the body.
Such expertise is a prized asset after the regulatory pathway was significantly derisked by FDA's recent decision on Aduhelm. Positive data at the Phase 2 level can set a company on a path to such approval. As investors are aware, an accelerated pathway approval is based on meeting surrogate or secondary endpoints, like amyloid reduction, followed by confirmatory studies later which can show disease modification.
Prothena has a broad pipeline of neurodegenerative therapeutics which makes it quite a unique smallcap company. Further catalysts include the Phase 2b initiation of the Parkinson's drug Prasinezumab, Phase 3 initiation of AL Amyloidosis drug Birtamimab, and other program updates. Later this year, Eli Lilly files a BLA for its drug Donanemab, which is similar to Aduhelm, that will draw attention to neurodegenerative disease companies.
Neurology is a complicated field with many drug failures. Prothena faces the same risks. There is a broader industry risk lurking in the background of whether the FDA will continue to be consistent with the approach it took with Aduhelm's approval.
Prothena has sufficient resources to pursue its programs for at least 2 years with about $350 million of cash and equivalents as of March 31, 2020, boosted by a secondary offering. In addition, the company received $60 million last month from Roche and $80 million from Bristol Myers this month, as part of licensing agreements. Future secondary offerings may occur and will be opportunistic based on data releases.
We had discussed Prothena earlier in the article, 3 Neurology Stocks for Alzheimer's Disease. After the approval of Biogen's Aduhelm, Prothena is now well-positioned to potentially be one of the prime beneficiaries of the FDA's acceptance of the beta-amyloid hypothesis for accelerated approval. A recent price target of $75 by an investment firm analyst can be exceeded over the next 12 months if data continues to maintain its positive tone.
Due to the de-risking of the regulatory pathway, M&A activity in the biotech group will rise as additional data is released in the second half, confirming the promising pipelines of some of these companies.
A few attractive neurodegenerative focused companies, besides Prothena, include Denali Therapeutics (DNLI), Cassava Sciences (SAVA), Anavex Life Sciences (AVXL), Annovis Bio (ANVS), Arvinas (ARVN), Supernus Pharmaceuticals (SUPN), Inmune Bio (INMB), Biohaven Pharmaceutical (BHVN), Intra-Cellular Therapies (ITCI), Cortexyme (CRTX), and Karuna Therapeutics (KRTX). Some of these companies in the past or now can be part of the Prudent Biotech or the Prudent Healthcare model portfolios.
Biotechs Remain Promising
In the earlier article, Biotechs Ready to Roar, it was explained why biotechs can outperform the market going forward, particularly if the interest rates remain stable. The Nasdaq Biotech Index (IBB) as of June 1 was neutral for the year, the S&P Biotech Select Index (XBI) was down -9% for the year, while the Prudent Biotech model portfolio was up +8%. Based on the muted and lagging performance of the biotech indexes and a 3-month consolidation, there is a lot of room for biotechs to run as positive events accumulate.
It is not just the neurodegenerative companies that look promising but many across the biotech industry. A few of these include Moderna (MRNA), Intellia Therapeutics (NTLA), BioNTech (BNTX), Beam Therapeutics (BEAM), Vericel (VCEL), Pacific Biosciences (PACB), Arrowhead Pharmaceuticals (ARWR), Axsome Therapeutics (AXSM), and Celldex (CLDX). Healthcare investing, particularly biotechs, is volatile and high-risk. Investors should pursue a concrete investment strategy preferring a portfolio approach by investing in a basket of promising companies that can assist in managing risk and overcoming mistakes.
The article was first published on Seeking Alpha.